Third Early "Booster" Dose Strategy in France of bnt162b2 SARS-CoV-2 Vaccine in Allogeneic Hematopoietic Stem Cell Transplant Recipients Enhances Neutralizing Antibody Responses
- PMID: 36146735
- PMCID: PMC9506309
- DOI: 10.3390/v14091928
Third Early "Booster" Dose Strategy in France of bnt162b2 SARS-CoV-2 Vaccine in Allogeneic Hematopoietic Stem Cell Transplant Recipients Enhances Neutralizing Antibody Responses
Abstract
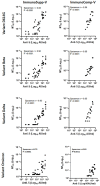
Immunocompromised individuals generally fail to mount efficacious immune humoral responses following vaccination. The emergence of SARS-CoV-2 variants of concern has raised the question as to whether levels of anti-spike protein antibodies achieved after two or three doses of the vaccine efficiently protect against breakthrough infection in the context of immune suppression. We used a fluorescence-based neutralization assay to test the sensitivity of SARS-CoV-2 variants (ancestral variant, Beta, Delta, and Omicron BA.1) to the neutralizing response induced by vaccination in highly immunosuppressed allogeneic HSCT recipients, tested after two and three doses of the BNT162b2 vaccine. We show that neutralizing antibody responses to the Beta and Delta variants in most immunocompromised HSCT recipients increased after three vaccine doses up to values similar to those observed in twice-vaccinated healthy adults and were significantly lower against Omicron BA.1. Overall, neutralization titers correlated with the amount of anti-S-RBD antibodies measured by means of enzyme immunoassay, indicating that commercially available assays can be used to quantify the anti-S-RBD antibody response as a reliable surrogate marker of humoral immune protection in both immunocompetent and immunocompromised individuals. Our findings support the recommendation of additional early vaccine doses as a booster of humoral neutralizing activity against emerging variants, in HSCT immunocompromised patients. In the context of Omicron circulation, it further emphasizes the need for reinforcement of preventive measures including the administration of monoclonal antibodies in this high-risk population.
Keywords: COVID-19 vaccine; HSCT immunocompromised patients; SARS-CoV-2; neutralizing antibody; variant of concern.
Conflict of interest statement
S.F. has been an advisor and/or speaker for Abbott diagnostics, MSD, and Abbvie. J.-M.P. has served as an advisor and/or speaker for Abbvie, Gilead, Merck, Regulus, Assembly Biosciences, Arbutus, and Memo Therapeutics. The other authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

