SARS-CoV-2 Spike and Nucleocapsid Antibody Response in Vaccinated Croatian Healthcare Workers and Infected Hospitalized Patients: A Single Center Cohort Study
- PMID: 36146773
- PMCID: PMC9503044
- DOI: 10.3390/v14091966
SARS-CoV-2 Spike and Nucleocapsid Antibody Response in Vaccinated Croatian Healthcare Workers and Infected Hospitalized Patients: A Single Center Cohort Study
Abstract
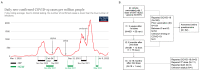
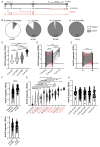
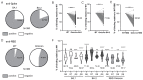
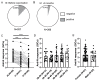
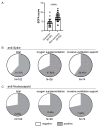
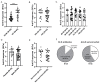
Studies assessing the dynamics and duration of antibody responses following SARS-CoV-2 infection or vaccination are an invaluable tool for vaccination schedule planning, assessment of risk groups and management of pandemics. In this study, we developed and employed ELISA assays to analyze the humoral responses to Nucleocapsid and Spike proteins in vaccinated health-care workers (HCW) and critically ill COVID-19 patients. Sera of more than 1000 HCWs and critically ill patients from the Clinical Hospital Center Rijeka were tested across a one-year period, encompassing the spread of major SARS-CoV-2 variants of concern (VOCs). We observed 97% of seroconversion in HCW cohort as well as sustained anti-Spike antibody response in vaccinees for more than 6 months. In contrast, the infection-induced anti-Nucleocapsid response was waning significantly in a six-month period. Furthermore, a substantial decrease in vaccinees' anti-Spike antibodies binding to Spike protein of Omicron VOC was also observed. Critically ill COVID-19 patients had higher levels of anti-Spike and anti-Nucleocapsid antibodies compared to HCWs. No significant differences in anti-Spike and anti-Nucleocapsid antibody levels between the critically ill COVID-19 patients that were on non-invasive oxygen supplementation and those on invasive ventilation support were observed. However, stronger anti-Spike, but not anti-Nucleocapsid, antibody response correlated with a better disease outcome in the cohort of patients on invasive ventilation support. Altogether, our results contribute to the growing pool of data on humoral responses to SARS-CoV-2 infection and vaccination.
Keywords: BNT162 vaccine; COVID-19; COVID-19 vaccine; Nucleocapsid; Omicron; SARS-CoV-2; Spike; antibody detection; healthcare workers; hospitalized patients.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hannah Ritchie E.M., Rodés-Guirao L., Appel C., Giattino C., Ortiz-Ospina E., Hasell J., Macdonald B., Beltekian D., Roser M. Coronavirus Pandemic (COVID-19) [(accessed on 20 June 2022)]. Available online: https://ourworldindata.org/coronavirus.
-
- Odak I., Schultze-Florey C.R., Hammerschmidt S.I., Ritter C., Willenzon S., Friedrichsen M., Ravens I., Sikora R., Bayir L.M., Gutierrez Jauregui R., et al. Longitudinal Tracking of Immune Responses in COVID-19 Convalescents Reveals Absence of Neutralization Activity against Omicron and Staggered Impairment to Other SARS-CoV-2 Variants of Concern. Front. Immunol. 2022;13:863039. doi: 10.3389/fimmu.2022.863039. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

