Apprehending the NAD+-ADPr-Dependent Systems in the Virus World
- PMID: 36146784
- PMCID: PMC9503650
- DOI: 10.3390/v14091977
Apprehending the NAD+-ADPr-Dependent Systems in the Virus World
Abstract
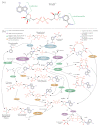
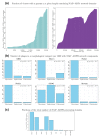
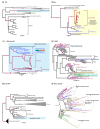
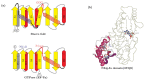
NAD+ and ADP-ribose (ADPr)-containing molecules are at the interface of virus-host conflicts across life encompassing RNA processing, restriction, lysogeny/dormancy and functional hijacking. We objectively defined the central components of the NAD+-ADPr networks involved in these conflicts and systematically surveyed 21,191 completely sequenced viral proteomes representative of all publicly available branches of the viral world to reconstruct a comprehensive picture of the viral NAD+-ADPr systems. These systems have been widely and repeatedly exploited by positive-strand RNA and DNA viruses, especially those with larger genomes and more intricate life-history strategies. We present evidence that ADP-ribosyltransferases (ARTs), ADPr-targeting Macro, NADAR and Nudix proteins are frequently packaged into virions, particularly in phages with contractile tails (Myoviruses), and deployed during infection to modify host macromolecules and counter NAD+-derived signals involved in viral restriction. Genes encoding NAD+-ADPr-utilizing domains were repeatedly exchanged between distantly related viruses, hosts and endo-parasites/symbionts, suggesting selection for them across the virus world. Contextual analysis indicates that the bacteriophage versions of ADPr-targeting domains are more likely to counter soluble ADPr derivatives, while the eukaryotic RNA viral versions might prefer macromolecular ADPr adducts. Finally, we also use comparative genomics to predict host systems involved in countering viral ADP ribosylation of host molecules.
Keywords: ADP-ribose; ADP-ribosyltransferase; NADase; RNA polymerase; RNA repair; anti-phage systems; cyclic ADP-ribose; nicotinamide adenine dinucleotide; nucleotides; sirtuin; virus evolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Identification of novel components of NAD-utilizing metabolic pathways and prediction of their biochemical functions.Mol Biosyst. 2012 Jun;8(6):1661-77. doi: 10.1039/c2mb05487f. Epub 2012 Mar 7. Mol Biosyst. 2012. PMID: 22399070
-
Phages reconstitute NAD+ to counter bacterial immunity.Nature. 2024 Oct;634(8036):1160-1167. doi: 10.1038/s41586-024-07986-w. Epub 2024 Sep 25. Nature. 2024. PMID: 39322677
-
Coronavirus infection and PARP expression dysregulate the NAD metabolome: An actionable component of innate immunity.J Biol Chem. 2020 Dec 25;295(52):17986-17996. doi: 10.1074/jbc.RA120.015138. Epub 2020 Oct 13. J Biol Chem. 2020. PMID: 33051211 Free PMC article.
-
The natural history of ADP-ribosyltransferases and the ADP-ribosylation system.Curr Top Microbiol Immunol. 2015;384:3-32. doi: 10.1007/82_2014_414. Curr Top Microbiol Immunol. 2015. PMID: 25027823 Free PMC article. Review.
-
Insights into the biogenesis, function, and regulation of ADP-ribosylation.Nat Chem Biol. 2018 Feb 14;14(3):236-243. doi: 10.1038/nchembio.2568. Nat Chem Biol. 2018. PMID: 29443986 Free PMC article. Review.
Cited by
-
Zinc-finger PARP proteins ADP-ribosylate alphaviral proteins and are required for interferon-γ-mediated antiviral immunity.Sci Adv. 2025 Jan 31;11(5):eadm6812. doi: 10.1126/sciadv.adm6812. Epub 2025 Jan 31. Sci Adv. 2025. PMID: 39888989 Free PMC article.
-
A ~40-kb flavi-like virus does not encode a known error-correcting mechanism.Proc Natl Acad Sci U S A. 2024 Jul 23;121(30):e2403805121. doi: 10.1073/pnas.2403805121. Epub 2024 Jul 17. Proc Natl Acad Sci U S A. 2024. PMID: 39018195 Free PMC article.
-
Reappraisal of the DNA phosphorothioate modification machinery: uncovering neglected functional modalities and identification of new counter-invader defense systems.Nucleic Acids Res. 2024 Feb 9;52(3):1005-1026. doi: 10.1093/nar/gkad1213. Nucleic Acids Res. 2024. PMID: 38163645 Free PMC article.
-
Comparative genomics of the Neodiprion sertifer nucleopolyhedrovirus from Turkey with the fewest ORFs among baculoviruses.Virus Genes. 2024 Apr;60(2):194-207. doi: 10.1007/s11262-024-02050-1. Epub 2024 Jan 19. Virus Genes. 2024. PMID: 38240955
-
Giant RNA genomes: Roles of host, translation elongation, genome architecture, and proteome in nidoviruses.Proc Natl Acad Sci U S A. 2025 Feb 18;122(7):e2413675122. doi: 10.1073/pnas.2413675122. Epub 2025 Feb 10. Proc Natl Acad Sci U S A. 2025. PMID: 39928875 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

