Human Cytomegalovirus Induces Vitamin-D Resistance In Vitro by Dysregulating the Transcriptional Repressor Snail
- PMID: 36146811
- PMCID: PMC9505537
- DOI: 10.3390/v14092004
Human Cytomegalovirus Induces Vitamin-D Resistance In Vitro by Dysregulating the Transcriptional Repressor Snail
Abstract
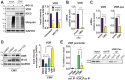
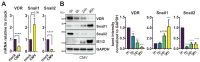
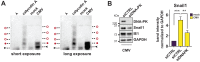
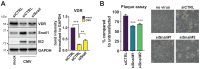
Vitamin-D supplementation is considered to play a beneficial role against multiple viruses due to its immune-regulating and direct antimicrobial effects. In contrast, the human cytomegalovirus (HCMV) has shown to be resistant to treatment with vitamin D in vitro by downregulation of the vitamin-D receptor. In this study, we aimed to elucidate the mechanism and possible biological consequences of vitamin-D resistance during HCMV infection. Mechanistically, HCMV induced vitamin-D resistance by downregulating the vitamin-D receptor (VDR) within hours of lytic infection. We found that the VDR was inhibited at the promoter level, and treatment with histone deacetylase inhibitors could restore VDR expression. VDR downregulation highly correlated with the upregulation of the transcriptional repressor Snail1, a mechanism likely contributing to the epigenetic inactivation of the VDR promoter, since siRNA-mediated knockdown of Snail partly restored levels of VDR expression. Finally, we found that direct addition of the vitamin-D-inducible antimicrobial peptide LL-37 strongly and significantly reduced viral titers in infected fibroblasts, highlighting VDR biological relevance and the potential of vitamin-D-inducible peptides for the antiviral treatment of vitamin-D deficient patients.
Keywords: HCMV; LL-37; Snail; VDR; calcitriol; cathelicidin; cytomegalovirus; vitamin D.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Epigenetic Modulation of Human Podocyte Vitamin D Receptor in HIV Milieu.J Mol Biol. 2015 Oct 9;427(20):3201-3215. doi: 10.1016/j.jmb.2015.07.011. Epub 2015 Jul 22. J Mol Biol. 2015. PMID: 26210663 Free PMC article.
-
The transcription factors Snail1 and Snail2 repress vitamin D receptor during colon cancer progression.J Steroid Biochem Mol Biol. 2010 Jul;121(1-2):106-9. doi: 10.1016/j.jsbmb.2010.01.014. Epub 2010 Feb 6. J Steroid Biochem Mol Biol. 2010. PMID: 20138990
-
Elevated vitamin D receptor levels in genetic hypercalciuric stone-forming rats are associated with downregulation of Snail.J Bone Miner Res. 2010 Apr;25(4):830-40. doi: 10.1359/jbmr.091010. J Bone Miner Res. 2010. PMID: 19929616 Free PMC article.
-
SNAIL vs vitamin D receptor expression in colon cancer: therapeutics implications.Br J Cancer. 2005 Mar 28;92(6):985-9. doi: 10.1038/sj.bjc.6602484. Br J Cancer. 2005. PMID: 15770204 Free PMC article. Review.
-
Emerging Roles of Vitamin D-Induced Antimicrobial Peptides in Antiviral Innate Immunity.Nutrients. 2022 Jan 11;14(2):284. doi: 10.3390/nu14020284. Nutrients. 2022. PMID: 35057465 Free PMC article. Review.
Cited by
-
The vitamin D receptor is essential for the replication of pseudorabies virus.mBio. 2024 Dec 11;15(12):e0213724. doi: 10.1128/mbio.02137-24. Epub 2024 Oct 30. mBio. 2024. PMID: 39475231 Free PMC article.
-
Immune Modulatory Effects of Vitamin D on Herpesvirus Infections.Int J Mol Sci. 2025 Feb 19;26(4):1767. doi: 10.3390/ijms26041767. Int J Mol Sci. 2025. PMID: 40004230 Free PMC article. Review.
-
Cytomegalovirus results in poor graft function via bone marrow-derived endothelial progenitor cells.Front Microbiol. 2024 Sep 18;15:1463335. doi: 10.3389/fmicb.2024.1463335. eCollection 2024. Front Microbiol. 2024. PMID: 39360328 Free PMC article.
References
-
- Bhalla A.K., Amento E.P., Serog B., Glimcher L.H. 1,25-Dihydroxyvitamin D3 inhibits antigen-induced T cell activation. J. Immunol. 1984;133:1748–1754. - PubMed
-
- Wang T.-T., Nestel F.P., Bourdeau V., Nagai Y., Wang Q., Liao J., Tavera-Mendoza L., Lin R., Hanrahan J.W., Mader S., et al. Cutting Edge: 1,25-Dihydroxyvitamin D 3 Is a Direct Inducer of Antimicrobial Peptide Gene Expression. J. Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. - DOI - PubMed
-
- Gordon Y.J., Huang L.C., Romanowski E.G., Yates K.A., Proske R.J., McDermott A.M. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr. Eye Res. 2005;30:385–394. doi: 10.1080/02713680590934111. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

