The ORF45 Protein of Kaposi's Sarcoma-Associated Herpesvirus and Its Critical Role in the Viral Life Cycle
- PMID: 36146816
- PMCID: PMC9506158
- DOI: 10.3390/v14092010
The ORF45 Protein of Kaposi's Sarcoma-Associated Herpesvirus and Its Critical Role in the Viral Life Cycle
Abstract
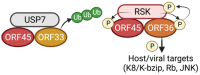
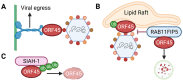
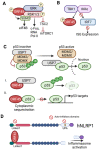
Kaposi's sarcoma-associated herpesvirus (KSHV) protein ORF45 is a virion-associated tegument protein that is unique to the gammaherpesvirus family. Generation of KSHV ORF45-knockout mutants and their subsequent functional analyses have permitted a better understanding of ORF45 and its context-specific and vital role in the KSHV lytic cycle. ORF45 is a multifaceted protein that promotes infection at both the early and late phases of the viral life cycle. As an immediate-early protein, ORF45 is expressed within hours of KSHV lytic reactivation and plays an essential role in promoting the lytic cycle, using multiple mechanisms, including inhibition of the host interferon response. As a tegument protein, ORF45 is necessary for the proper targeting of the viral capsid for envelopment and release, affecting the late stage of the viral life cycle. A growing list of ORF45 interaction partners have been identified, with one of the most well-characterized being the association of ORF45 with the host extracellular-regulated kinase (ERK) p90 ribosomal s6 kinase (RSK) signaling cascade. In this review, we describe ORF45 expression kinetics, as well as the host and viral interaction partners of ORF45 and the significance of these interactions in KSHV biology. Finally, we discuss the role of ORF45 homologs in gammaherpesvirus infections.
Keywords: KSHV; MAP kinase pathway; ORF45; RSK; gammaherpesvirus; herpesvirus; immediate-early gene; interferon; tegument.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Development of an ORF45-Derived Peptide To Inhibit the Sustained RSK Activation and Lytic Replication of Kaposi's Sarcoma-Associated Herpesvirus.J Virol. 2019 May 1;93(10):e02154-18. doi: 10.1128/JVI.02154-18. Print 2019 May 15. J Virol. 2019. PMID: 30842327 Free PMC article.
-
Activation of p90 ribosomal S6 kinases by ORF45 of Kaposi's sarcoma-associated herpesvirus is critical for optimal production of infectious viruses.J Virol. 2015 Jan;89(1):195-207. doi: 10.1128/JVI.01937-14. Epub 2014 Oct 15. J Virol. 2015. PMID: 25320298 Free PMC article.
-
Phosphoproteomic Analysis of KSHV-Infected Cells Reveals Roles of ORF45-Activated RSK during Lytic Replication.PLoS Pathog. 2015 Jul 2;11(7):e1004993. doi: 10.1371/journal.ppat.1004993. eCollection 2015 Jul. PLoS Pathog. 2015. PMID: 26133373 Free PMC article.
-
ORF45, a multifunctional immediate early and tegument protein of KSHV.J Med Virol. 2023 Mar;95(3):e28659. doi: 10.1002/jmv.28659. J Med Virol. 2023. PMID: 36905218 Review.
-
Regulation of KSHV Latency and Lytic Reactivation.Viruses. 2020 Sep 17;12(9):1034. doi: 10.3390/v12091034. Viruses. 2020. PMID: 32957532 Free PMC article. Review.
Cited by
-
Conserved linear motif within the immediate early protein ORF45 promotes its engagement with KSHV lytic cycle-promoting forkhead transcription factors, FOXK1 and FOXK2.J Virol. 2024 Oct 22;98(10):e0088624. doi: 10.1128/jvi.00886-24. Epub 2024 Sep 17. J Virol. 2024. PMID: 39287387 Free PMC article.
-
Hypoxia and hypoxia-inducible factors in Kaposi sarcoma-associated herpesvirus infection and disease pathogenesis.J Med Virol. 2023 Sep;95(9):e29071. doi: 10.1002/jmv.29071. J Med Virol. 2023. PMID: 37665216 Free PMC article. Review.
-
Kaposi's Sarcoma-Associated Herpesvirus Immediate Early Proteins Trigger FOXQ1 Expression in Oral Epithelial Cells, Engaging in a Novel Lytic Cycle-Sustaining Positive Feedback Loop.J Virol. 2023 Mar 30;97(3):e0169622. doi: 10.1128/jvi.01696-22. Epub 2023 Feb 23. J Virol. 2023. PMID: 36815831 Free PMC article.
-
Study of alloferon, a novel immunomodulatory antimicrobial peptide (AMP), and its analogues.Front Pharmacol. 2024 Feb 16;15:1359261. doi: 10.3389/fphar.2024.1359261. eCollection 2024. Front Pharmacol. 2024. PMID: 38434708 Free PMC article. Review.
-
Characterization of BoHV-4 ORF45.Front Microbiol. 2023 May 10;14:1171770. doi: 10.3389/fmicb.2023.1171770. eCollection 2023. Front Microbiol. 2023. PMID: 37234529 Free PMC article.
References
-
- Soulier J., Grollet L., Oksenhendler E., Cacoub P., Cazals-Hatem D., Babinet P., d’Agay M.F., Clauvel J.P., Raphael M., Degos L. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276–1280. doi: 10.1182/blood.V86.4.1276.bloodjournal8641276. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

