Human Cytomegalovirus Modifies Placental Small Extracellular Vesicle Composition to Enhance Infection of Fetal Neural Cells In Vitro
- PMID: 36146834
- PMCID: PMC9501265
- DOI: 10.3390/v14092030
Human Cytomegalovirus Modifies Placental Small Extracellular Vesicle Composition to Enhance Infection of Fetal Neural Cells In Vitro
Abstract
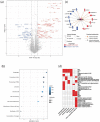
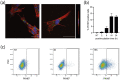
Although placental small extracellular vesicles (sEVs) are extensively studied in the context of pregnancy, little is known about their role during viral congenital infection, especially at the beginning of pregnancy. In this study, we examined the consequences of human cytomegalovirus (hCMV) infection on sEVs production, composition, and function using an immortalized human cytotrophoblast cell line derived from first trimester placenta. By combining complementary approaches of biochemistry, electron microscopy, and quantitative proteomic analysis, we showed that hCMV infection increases the yield of sEVs produced by cytotrophoblasts and modifies their protein content towards a potential proviral phenotype. We further demonstrate that sEVs secreted by hCMV-infected cytotrophoblasts potentiate infection in naive recipient cells of fetal origin, including human neural stem cells. Importantly, these functional consequences are also observed with sEVs prepared from an ex vivo model of infected histocultures from early placenta. Based on these findings, we propose that placental sEVs could be important actors favoring viral dissemination to the fetal brain during hCMV congenital infection.
Keywords: congenital infection; cytotrophoblast; extracellular vesicles; hCMV; placenta.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Pereira L., Maidji E., Fisher S.J., McDonagh S., Tabata T. HCMV persistence in the population: Potential transplacental transmission. In: Arvin A., Campadelli-Fiume G., Mocarski E., Moore P.S., Roizman B., Whitley R., Yamanishi K., editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge University Press; Cambridge, UK: 2007. - PubMed
-
- Tabata T., Petitt M., Fang-Hoover J., Rivera J., Nozawa N., Shiboski S., Inoue N., Pereira L. Cytomegalovirus Impairs Cytotrophoblast-Induced Lymphangiogenesis and Vascular Remodeling in an In Vivo Human Placentation Model. Am. J. Pathol. 2012;181:1540–1559. doi: 10.1016/j.ajpath.2012.08.003. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

