SARS-CoV-2 Seroprevalence Study in Pediatric Patients and Health Care Workers Using Multiplex Antibody Immunoassays
- PMID: 36146844
- PMCID: PMC9502584
- DOI: 10.3390/v14092039
SARS-CoV-2 Seroprevalence Study in Pediatric Patients and Health Care Workers Using Multiplex Antibody Immunoassays
Abstract
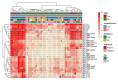
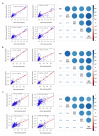
SARS-CoV-2 infection has become a global health problem specially exacerbated with the continuous appearance of new variants. Healthcare workers (HCW) have been one of the most affected sectors. Children have also been affected, and although infection generally presents as a mild disease, some have developed the Pediatric Inflammatory Multisystem Syndrome Temporally Associated with SARS-CoV-2 (PIMS-TS). We recruited 190 adults (HCW and cohabitants, April to June 2020) and 57 children (April 2020 to September 2021), of whom 12 developed PIMS-TS, in a hospital-based study in Spain. Using an in-house Luminex assay previously validated, antibody levels were measured against different spike and nucleocapsid SARS-CoV-2 proteins, including the receptor-binding domain (RBD) of the Alpha, Beta, Gamma, and Delta variants of concern (VoC). Seropositivity rates obtained from children and adults, respectively, were: 49.1% and 11% for IgG, 45.6% and 5.8% for IgA, and 35.1% and 7.3% for IgM. Higher antibody levels were detected in children who developed PIMS-TS compared to those who did not. Using the COVID-19 IgM/IgA ELISA (Vircell, S.L.) kit, widely implemented in Spanish hospitals, a high number of false positives and lower seroprevalences compared with the Luminex estimates were found, indicating a significantly lower specificity and sensitivity. Comparison of antibody levels against RBD-Wuhan versus RBD-VoCs indicated that the strongest positive correlations for all three isotypes were with RBD-Alpha, while the lowest correlations were with RBD-Delta for IgG, RBD-Gamma for IgM, and RBD-Beta for IgA. This study highlights the differences in antibody levels between groups with different demographic and clinical characteristics, as well as reporting the IgG, IgM, and IgA response to RBD VoC circulating at the study period.
Keywords: COVID-19; PIMS-TS; SARS-CoV-2; antibody; antigen; children; cohort; healthcare workers; seropositivity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients.Sci Immunol. 2020 Oct 8;5(52):eabe0367. doi: 10.1126/sciimmunol.abe0367. Sci Immunol. 2020. PMID: 33033172 Free PMC article.
-
SARS-CoV-2-Specific Antibody Detection for Seroepidemiology: A Multiplex Analysis Approach Accounting for Accurate Seroprevalence.J Infect Dis. 2020 Oct 1;222(9):1452-1461. doi: 10.1093/infdis/jiaa479. J Infect Dis. 2020. PMID: 32766833 Free PMC article.
-
Distinct anti-NP, anti-RBD and anti-Spike antibody profiles discriminate death from survival in COVID-19.Front Immunol. 2023 Oct 9;14:1206979. doi: 10.3389/fimmu.2023.1206979. eCollection 2023. Front Immunol. 2023. PMID: 37876932 Free PMC article.
-
PIMS-TS, the New Paediatric Systemic Inflammatory Disease Related to Previous Exposure to SARS-CoV-2 Infection-"Rheumatic Fever" of the 21st Century?Int J Mol Sci. 2021 Apr 26;22(9):4488. doi: 10.3390/ijms22094488. Int J Mol Sci. 2021. PMID: 33925779 Free PMC article. Review.
-
COVID-19 in children. II: Pathogenesis, disease spectrum and management.J Paediatr Child Health. 2022 Jan;58(1):46-53. doi: 10.1111/jpc.15811. Epub 2021 Oct 25. J Paediatr Child Health. 2022. PMID: 34694037 Free PMC article. Review.
Cited by
-
MultiSero: An Open-Source Multiplex-ELISA Platform for Measuring Antibody Responses to Infection.Pathogens. 2023 May 2;12(5):671. doi: 10.3390/pathogens12050671. Pathogens. 2023. PMID: 37242341 Free PMC article.
-
SARS-CoV-2 Antibody Responses in Pediatric Patients: A Bibliometric Analysis.Biomedicines. 2023 May 16;11(5):1455. doi: 10.3390/biomedicines11051455. Biomedicines. 2023. PMID: 37239126 Free PMC article. Review.
-
Seroprevalence of SARS-CoV-2 infection in pediatric patients in a tertiary care hospital setting.PLoS One. 2024 Sep 24;19(9):e0310860. doi: 10.1371/journal.pone.0310860. eCollection 2024. PLoS One. 2024. PMID: 39316628 Free PMC article.
-
Variant-specific antibody profiling for tracking SARS-CoV-2 variant infections in children and adolescents.Front Immunol. 2024 Aug 27;15:1434291. doi: 10.3389/fimmu.2024.1434291. eCollection 2024. Front Immunol. 2024. PMID: 39257574 Free PMC article.

