RIOK3 and Its Alternatively Spliced Isoform Have Disparate Roles in the Innate Immune Response to Rift Valley Fever Virus (MP12) Infection
- PMID: 36146870
- PMCID: PMC9502082
- DOI: 10.3390/v14092064
RIOK3 and Its Alternatively Spliced Isoform Have Disparate Roles in the Innate Immune Response to Rift Valley Fever Virus (MP12) Infection
Abstract
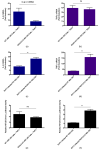
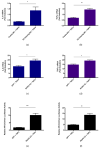
Rift Valley fever virus (RVFV) is a pathogenic human and livestock RNA virus that poses a significant threat to public health and biosecurity. During RVFV infection, the atypical kinase RIOK3 plays important roles in the innate immune response. Although its exact functions in innate immunity are not completely understood, RIOK3 has been shown to be necessary for mounting an antiviral interferon (IFN) response to RVFV in epithelial cells. Furthermore, after immune stimulation, the splicing pattern for RIOK3 mRNA changes markedly, and RIOK3's dominant alternatively spliced isoform, RIOK3 X2, exhibits an opposite effect on the IFN response by dampening it. Here, we further investigate the roles of RIOK3 and its spliced isoform in other innate immune responses to RVFV, namely the NFκB-mediated inflammatory response. We find that while RIOK3 is important for negatively regulating this inflammatory pathway, its alternatively spliced isoform, RIOK3 X2, stimulates it. Overall, these data demonstrate that both RIOK3 and its X2 isoform have unique roles in separate innate immune pathways that respond to RVFV infection.
Keywords: NFκB; RIOK3; Rift Valley fever virus; alternative splicing; innate immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Alternative Splicing of RIOK3 Engages the Noncanonical NFκB Pathway during Rift Valley Fever Virus Infection.Viruses. 2023 Jul 18;15(7):1566. doi: 10.3390/v15071566. Viruses. 2023. PMID: 37515252 Free PMC article.
-
Tra2beta-Dependent Regulation of RIO Kinase 3 Splicing During Rift Valley Fever Virus Infection Underscores the Links Between Alternative Splicing and Innate Antiviral Immunity.Front Cell Infect Microbiol. 2022 Jan 19;11:799024. doi: 10.3389/fcimb.2021.799024. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35127560 Free PMC article.
-
The Atypical Kinase RIOK3 Limits RVFV Propagation and Is Regulated by Alternative Splicing.Viruses. 2021 Feb 26;13(3):367. doi: 10.3390/v13030367. Viruses. 2021. PMID: 33652597 Free PMC article.
-
Arm race between Rift Valley fever virus and host.Front Immunol. 2022 Dec 22;13:1084230. doi: 10.3389/fimmu.2022.1084230. eCollection 2022. Front Immunol. 2022. PMID: 36618346 Free PMC article. Review.
-
Single-cycle replicable Rift Valley fever virus mutants as safe vaccine candidates.Virus Res. 2016 May 2;216:55-65. doi: 10.1016/j.virusres.2015.05.012. Epub 2015 May 27. Virus Res. 2016. PMID: 26022573 Free PMC article. Review.
Cited by
-
Genomic Regions Associated with Resistance to Gastrointestinal Nematode Parasites in Sheep-A Review.Genes (Basel). 2024 Jan 30;15(2):187. doi: 10.3390/genes15020187. Genes (Basel). 2024. PMID: 38397178 Free PMC article. Review.
-
RNA virus infections and their effect on host alternative splicing.Antiviral Res. 2023 Feb;210:105503. doi: 10.1016/j.antiviral.2022.105503. Epub 2022 Dec 23. Antiviral Res. 2023. PMID: 36572191 Free PMC article. Review.
-
Alternative Splicing of RIOK3 Engages the Noncanonical NFκB Pathway during Rift Valley Fever Virus Infection.Viruses. 2023 Jul 18;15(7):1566. doi: 10.3390/v15071566. Viruses. 2023. PMID: 37515252 Free PMC article.
-
Illuminating the druggable genome: Pathways to progress.Drug Discov Today. 2024 Mar;29(3):103805. doi: 10.1016/j.drudis.2023.103805. Epub 2023 Oct 27. Drug Discov Today. 2024. PMID: 37890715 Free PMC article. Review.
-
Rift Valley Fever Virus-Infection, Pathogenesis and Host Immune Responses.Pathogens. 2023 Sep 19;12(9):1174. doi: 10.3390/pathogens12091174. Pathogens. 2023. PMID: 37764982 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

