COS-7 and SVGp12 Cellular Models to Study JCPyV Replication and MicroRNA Expression after Infection with Archetypal and Rearranged-NCCR Viral Strains
- PMID: 36146876
- PMCID: PMC9502812
- DOI: 10.3390/v14092070
COS-7 and SVGp12 Cellular Models to Study JCPyV Replication and MicroRNA Expression after Infection with Archetypal and Rearranged-NCCR Viral Strains
Abstract
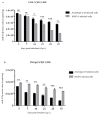
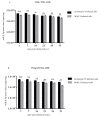
Since the non-coding control region (NCCR) and microRNA (miRNA) could represent two different and independent modalities of regulating JC polyomavirus (JCPyV) replication at the transcriptional and post-transcriptional levels, the interplay between JC viral load based on NCCR architecture and miRNA levels, following JCPyV infection with archetypal and rearranged (rr)-NCCR JCPyV variants, was explored in COS-7 and SVGp12 cells infected by different JCPyV strains. Specifically, the involvement of JCPyV miRNA in regulating viral replication was investigated for the archetypal CY strain-which is the transmissible form-and for the rearranged MAD-1 strain, which is the first isolated variant from patients with progressive multifocal leukoencephalopathy. The JCPyV DNA viral load was low in cells infected with CY compared with that in MAD-1-infected cells. Productive viral replication was observed in both cell lines. The expression of JCPyV miRNAs was observed from 3 days after viral infection in both cell types, and miR-J1-5p expression was inversely correlated with the JCPyV replication trend. The JCPyV miRNAs in the exosomes present in the supernatants produced by the infected cells could be carried into uninfected cells. Additional investigations of the expression of JCPyV miRNAs and their presence in exosomes are necessary to shed light on their regulatory role during viral reactivation.
Keywords: COS-7; JCPyV replication; NCCR viral strains; SVGp12; cellular models; exosomes; miRNA expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Should SVGp12 Be Used for JC Polyomavirus Studies? Comment on Prezioso et al. COS-7 and SVGp12 Cellular Models to Study JCPyV Replication and MicroRNA Expression after Infection with Archetypal and Rearranged-NCCR Viral Strains. Viruses 2022, 14, 2070.Viruses. 2022 Dec 29;15(1):89. doi: 10.3390/v15010089. Viruses. 2022. PMID: 36680132 Free PMC article.
-
Reply to Henriksen, S.; Rinaldo, C.H. Should SVGp12 Be Used for JC Polyomavirus Studies? Comment on "Prezioso et al. COS-7 and SVGp12 Cellular Models to Study JCPyV Replication and MicroRNA Expression after Infection with Archetypal and Rearranged-NCCR Viral Strains. Viruses 2022, 14, 2070".Viruses. 2022 Dec 29;15(1):93. doi: 10.3390/v15010093. Viruses. 2022. PMID: 36680133 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

