Structural Basis for the Inhibition of Coronaviral Main Proteases by a Benzothiazole-Based Inhibitor
- PMID: 36146880
- PMCID: PMC9505605
- DOI: 10.3390/v14092075
Structural Basis for the Inhibition of Coronaviral Main Proteases by a Benzothiazole-Based Inhibitor
Abstract
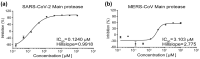
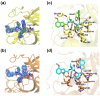
The ongoing spread of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has caused hundreds of millions of cases and millions of victims worldwide with serious consequences to global health and economies. Although many vaccines protecting against SARS-CoV-2 are currently available, constantly emerging new variants necessitate the development of alternative strategies for prevention and treatment of COVID-19. Inhibitors that target the main protease (Mpro) of SARS-CoV-2, an essential enzyme that promotes viral maturation, represent a key class of antivirals. Here, we showed that a peptidomimetic compound with benzothiazolyl ketone as warhead, YH-53, is an effective inhibitor of SARS-CoV-2, SARS-CoV, and MERS-CoV Mpros. Crystal structures of Mpros from SARS-CoV-2, SARS-CoV, and MERS-CoV bound to the inhibitor YH-53 revealed a unique ligand-binding site, which provides new insights into the mechanism of inhibition of viral replication. A detailed analysis of these crystal structures defined the key molecular determinants required for inhibition and illustrate the binding mode of Mpros from other coronaviruses. In consideration of the important role of Mpro in developing antivirals against coronaviruses, insights derived from this study should add to the design of pan-coronaviral Mpro inhibitors that are safer and more effective.
Keywords: YH-53; coronavirus; inhibitor; main protease; warhead.
Conflict of interest statement
Authors, Shenzhen Crystalo Biopharmaceutical Co., Ltd., and Jiangxi Jmerry Biopharmaceutical Co., Ltd. declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

