Pathogenicity of Avian Polyomaviruses and Prospect of Vaccine Development
- PMID: 36146885
- PMCID: PMC9505546
- DOI: 10.3390/v14092079
Pathogenicity of Avian Polyomaviruses and Prospect of Vaccine Development
Abstract
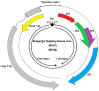
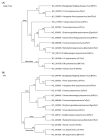
Polyomaviruses are nonenveloped icosahedral viruses with a double-stranded circular DNA containing approximately 5000 bp and 5-6 open reading frames. In contrast to mammalian polyomaviruses (MPVs), avian polyomaviruses (APVs) exhibit high lethality and multipathogenicity, causing severe infections in birds without oncogenicity. APVs are classified into 10 major species: Adélie penguin polyomavirus, budgerigar fledgling disease virus, butcherbird polyomavirus, canary polyomavirus, cormorant polyomavirus, crow polyomavirus, Erythrura gouldiae polyomavirus, finch polyomavirus, goose hemorrhagic polyomavirus, and Hungarian finch polyomavirus under the genus Gammapolyomavirus. This paper briefly reviews the genomic structure and pathogenicity of the 10 species of APV and some of their differences in terms of virulence from MPVs. Each gene's genomic size, number of amino acid residues encoding each gene, and key biologic functions are discussed. The rationale for APV classification from the Polyomavirdae family and phylogenetic analyses among the 10 APVs are also discussed. The clinical symptoms in birds caused by APV infection are summarized. Finally, the strategies for developing an effective vaccine containing essential epitopes for preventing virus infection in birds are discussed. We hope that more effective and safe vaccines with diverse protection will be developed in the future to solve or alleviate the problems of viral infection.
Keywords: Gammapolyomavirus; avian polyomavirus; genomic structure; pathogenicity; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Characterization of two novel polyomaviruses of birds by using multiply primed rolling-circle amplification of their genomes.J Virol. 2006 Apr;80(7):3523-31. doi: 10.1128/JVI.80.7.3523-3531.2006. J Virol. 2006. PMID: 16537620 Free PMC article.
-
Genetic diversity in twenty variants of the avian polyomavirus.Avian Dis. 1999 Apr-Jun;43(2):207-18. Avian Dis. 1999. PMID: 10396633
-
Butcherbird polyomavirus isolated from a grey butcherbird (Cracticus torquatus) in Queensland, Australia.Vet Microbiol. 2014 Jan 31;168(2-4):302-11. doi: 10.1016/j.vetmic.2013.11.026. Epub 2013 Nov 28. Vet Microbiol. 2014. PMID: 24355535
-
[New, newer, newest human polyomaviruses: how far?].Mikrobiyol Bul. 2013 Apr;47(2):362-81. doi: 10.5578/mb.5377. Mikrobiyol Bul. 2013. PMID: 23621738 Review. Turkish.
-
Polyomaviruses of nonhuman primates: implications for research.Comp Med. 2008 Feb;58(1):51-6. Comp Med. 2008. PMID: 19793457 Free PMC article. Review.
Cited by
-
Polyomaviruses and the risk of breast cancer: a systematic review and meta-analysis.Infect Agent Cancer. 2025 Mar 4;20(1):14. doi: 10.1186/s13027-025-00644-4. Infect Agent Cancer. 2025. PMID: 40038755 Free PMC article. Review.
-
Identification of avian polyomavirus and its pathogenicity to SPF chickens.Front Microbiol. 2024 Jan 3;14:1320264. doi: 10.3389/fmicb.2023.1320264. eCollection 2023. Front Microbiol. 2024. PMID: 38235429 Free PMC article.
-
Prevalence, genotypes, and infection risk factors of psittacine beak and feather disease virus and budgerigar fledgling disease virus in captive birds in Hong Kong.Arch Virol. 2024 Apr 5;169(5):91. doi: 10.1007/s00705-024-06017-3. Arch Virol. 2024. PMID: 38578455 Free PMC article.
-
Novel polyomavirus in the endangered garden dormouse Eliomys quercinus.Virol J. 2024 Nov 27;21(1):309. doi: 10.1186/s12985-024-02581-x. Virol J. 2024. PMID: 39605065 Free PMC article.
-
Structural similarity of human papillomavirus E4 and polyomaviral VP4 exhibited by genomic analysis of the common kestrel (Falco tinnunculus) polyomavirus.Vet Res Commun. 2024 Feb;48(1):309-315. doi: 10.1007/s11259-023-10210-1. Epub 2023 Sep 9. Vet Res Commun. 2024. PMID: 37688754 Free PMC article.
References
-
- Johne R., Paul G., Enderlein D., Stahl T., Grund C., Muller H. Avian polyomavirus mutants with deletions in the VP4-encoding region show deficiencies in capsid assembly and virus release, and have reduced infectivity in chicken. Pt 3J. Gen. Virol. 2007;88:823–830. doi: 10.1099/vir.0.82506-0. - DOI - PubMed
-
- Rott O., Kroger M., Muller H., Hobom G. The genome of budgerigar fledgling disease virus, an avian polyomavirus. [(accessed on 15 January 2022)];Virology. 1988 165:74–86. doi: 10.1016/0042-6822(88)90660-5. Available online: https://www.ncbi.nlm.nih.gov/pubmed/2838972. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

