Efficacy and Safety of Aflibercept Therapy for Diabetic Macular Edema: A Systematic Review and Meta-Analysis
- PMID: 36147265
- PMCID: PMC9486998
- DOI: 10.4103/joco.joco_308_21
Efficacy and Safety of Aflibercept Therapy for Diabetic Macular Edema: A Systematic Review and Meta-Analysis
Abstract
Purpose: To assess the real-world efficacy and safety of aflibercept for the treatment of diabetic macular edema (DME).
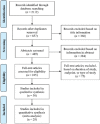
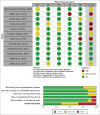
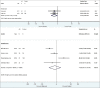
Methods: A systematic search was conducted across multiple databases. Articles were included if participants had DME and received aflibercept treatment for a minimum of 52 ± 4 weeks. Primary outcomes included changes in best-corrected visual acuity (BCVA) and central macular thickness (CMT). A risk of bias assessment of studies was completed, pooled estimates were obtained, and a meta-regression was performed. Information on adverse events was collected.
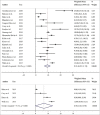
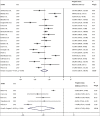
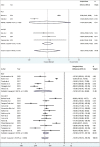
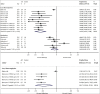
Results: The search yielded 2112 articles, of which 30 were included. Aflibercept was more effective than laser photocoagulation functionally (12-month BCVA-weighted mean difference [WMD] = 10.77 letters, P < 0.001; 24 months = 8.12 letters, P < 0.001) and anatomically (12-month CMT WMD = -114.12 μm, P < 0.001; 24 months = -90.4 μm, P = 0.004). Compared to bevacizumab, aflibercept was noninferior at improving BCVA at 12 months (WMD = 1.71 letters, P = 0.34) and 24 months (WMD = 1.58 letters, P = 0.083). One study found that aflibercept was more effective than bevacizumab anatomically at 1 and 2 years (P < 0.001 at 12 and 24 months). Compared to ranibizumab, aflibercept rendered a greater improvement in BCVA at 1 year (WMD = 1.76 letters, P = 0.001), but not 2 years (WMD = 1.66 letters, P = 0.072). CMT was not significantly different between both therapies at 12 months (WMD = -14.30 μm, P = 0.282) and 24 months (P = 0.08). One study reported greater functional improvement with aflibercept compared with dexamethasone (P = 0.004), but inferiority in reducing CMT (P < 0.001). Meta-regression analysis demonstrated that dosing schedule was found to impact outcomes at 12 and 24 months, while study design and sample size did not impact outcomes at 12 months. There were minimal safety concerns using aflibercept therapy.
Conclusions: Aflibercept is a safe and effective therapy option for DME in the clinical setting, performing superiorly to laser photocoagulation. Evidence regarding comparisons with bevacizumab, ranibizumab, and dexamethasone is mixed and limited.
Keywords: Aflibercept; Antivascular endothelial growth factor; Diabetic macular edema; Eylea; Retina.
Copyright: © 2022 Journal of Current Ophthalmology.
Conflict of interest statement
There are no conflicts of interest.
Figures






References
-
- Ding J, Wong TY. Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr Diab Rep. 2012;12:346–54. - PubMed
-
- Kim EJ, Lin WV, Rodriguez SM, Chen A, Loya A, Weng CY. Treatment of diabetic macular edema. Curr Diab Rep. 2019;19:68. - PubMed
-
- Canada H, editor. Health Canada. Avastin. Drug Product Database. Ottawa, Ontario, Canada: Health Canada; 2005.
-
- Lucentis. Government of Canada; 2007. [Last accessed on 2021 Jul 10]. Available from: https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=78217 .
Publication types
LinkOut - more resources
Full Text Sources
