Muscarinic receptor M3 activation promotes fibrocytes contraction
- PMID: 36147316
- PMCID: PMC9485632
- DOI: 10.3389/fphar.2022.939780
Muscarinic receptor M3 activation promotes fibrocytes contraction
Abstract
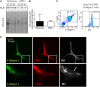
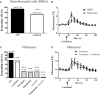
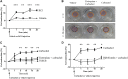
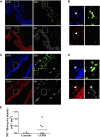
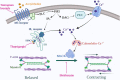
Fibrocytes are monocyte-derived cells able to differentiate into myofibroblasts-like cells. We have previously shown that they are increased in the bronchi of Chronic Obstructive Pulmonary Disease (COPD) patients and associated to worse lung function. COPD is characterized by irreversible airflow obstruction, partly due to an increased cholinergic environment. Our goal was to investigate muscarinic signalling in COPD fibrocytes. Fibrocytes were isolated from 16 patients with COPD's blood and presence of muscarinic M3 receptor was assessed at the transcriptional and protein levels. Calcium signalling and collagen gels contraction experiments were performed in presence of carbachol (cholinergic agonist) ± tiotropium bromide (antimuscarinic). Expression of M3 receptor was confirmed by Western blot and flow cytometry in differentiated fibrocytes. Immunocytochemistry showed the presence of cytoplasmic and membrane-associated pools of M3. Stimulation with carbachol elicited an intracellular calcium response in 35.7% of fibrocytes. This response was significantly blunted by the presence of tiotropium bromide: 14.6% of responding cells (p < 0.0001). Carbachol induced a significant contraction of fibrocytes embedded in collagen gels (13.6 ± 0.3% versus 2.5 ± 4.1%; p < 0.0001), which was prevented by prior tiotropium bromide addition (4.1 ± 2.7% of gel contraction; p < 0.0001). Finally, M3-expressing fibrocytes were also identified in situ in the peri-bronchial area of COPD patients' lungs, and there was a tendency to an increased density compared to healthy patient's lungs. In conclusion, around 1/3 of COPD patients' fibrocytes express a functional muscarinic M3 receptor. Cholinergic-induced fibrocyte contraction might participate in airway diameter reduction and subsequent increase of airflow resistance in patients with COPD. The inhibition of these processes could participate to the beneficial effects of muscarinic antagonists for COPD treatment.
Keywords: COPD; M3; cholinergic; contraction; fibrocyte.
Copyright © 2022 Henrot, Eyraud, Maurat, Point, Cardouat, Quignard, Esteves, Trian, Girodet, Marthan, Zysman, Berger and Dupin.
Conflict of interest statement
PH, EE, EM, SP, GC, JFQ, PE, TT and RM report no relevant conflict of interest; ID, P-OG and PB report a patent (EP N3050574: Use of plerixafor for treating and/or preventing acute exacerbations of chronic obstructive pulmonary disease (Berger et al., 2019)) (granted); POG reports grants, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from Chiesi, personal fees and non-financial support from GlaxoSmithKline, personal fees and non-financial support from Novartis, personal fees and non-financial support from Sanofi, outside the submitted work; MZ reports grants from AstraZeneca; grants “Fondation Bordeaux Université,” with funding from “Assistance Ventilatoire à Domicile” (AVAD) and “Fédération Girondine de Lutte contre les Maladies Respiratoires” (FGLMR) and personal fees from AstraZeneca, Boehringer Ingelheim, Novartis, Chiesi, GlaxoSmithKline and non-financial support Lilly outside the submitted work; PB reports grants from AstraZeneca, Glaxo-Smith-Kline, Novartis, Chiesi, which support COBRA during the conduct of the study; grants and personal fees from AstraZeneca, BoehringerIngelheim, Novartis, personal fees and non-financial support from Chiesi, Sanofi, Menarini, outside the submitted work; ID reports grant from the “Fondation Bordeaux Université,” with funding from “Assistance Ventilatoire à Domicile” (AVAD) and “Fédération Girondine de Lutte contre les Maladies Respiratoires” (FGLMR) during the conduct of the study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Barnes P. J. (1993). Muscarinic receptor subtypes in airways. Eur. Respir. J. 6, 328–331. - PubMed
LinkOut - more resources
Full Text Sources

