Functional characterization of cannabidiol effect on the serotonergic neurons of the dorsal raphe nucleus in rat brain slices
- PMID: 36147343
- PMCID: PMC9485894
- DOI: 10.3389/fphar.2022.956886
Functional characterization of cannabidiol effect on the serotonergic neurons of the dorsal raphe nucleus in rat brain slices
Abstract
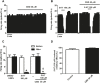
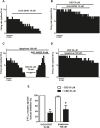
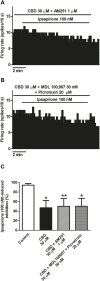
Cannabidiol (CBD), the main non-psychoactive cannabinoid found in the cannabis plant, elicits several pharmacological effects via the 5-HT1A receptor. The dorsal raphe nucleus (DRN) is the main serotonergic cluster in the brain that expresses the 5-HT1A receptor. To date, the effect of CBD on the neuronal activity of DRN 5-HT cells and its interaction with somatodendritic 5-HT1A autoreceptors have not been characterized. Our aim was to study the effect of CBD on the firing activity of DRN 5-HT cells and the 5-HT1A autoreceptor activation by electrophysiological and calcium imaging techniques in male Sprague-Dawley rat brain slices. Perfusion with CBD (30 μM, 10 min) did not significantly change the firing rate of DRN 5-HT cells or the inhibitory effect of 5-HT (50-100 μM, 1 min). However, in the presence of CBD (30 μM, 10 min), the inhibitory effects of 8-OH-DPAT (10 nM) and ipsapirone (100 nM) were reduced by 66% and 53%, respectively. CBD failed to reverse ipsapirone-induced inhibition, whereas perfusion with the 5-HT1A receptor antagonist WAY100635 (30 nM) completely restored by 97.05 ± 14.63% the firing activity of 5-HT cells. Administration of AM251 (1 µM), MDL100907 (30 nM), or picrotoxin (20 μM) did not change the blockade produced by CBD (30 μM) on ipsapirone-induced inhibition. Our study also shows that CBD failed to modify the KCl (15 mM, 4 min)-evoked increase in [Ca2+]i or the inhibitory effect of ipsapirone (1 μM, 4 min) on KCl-evoked [Ca2+]i. In conclusion, CBD does not activate 5-HT1A autoreceptors, but it hindered the inhibitory effect produced by selective 5-HT1A receptor agonists on the firing activity of DRN 5-HT cells through a mechanism that does not involve CB1, 5-HT2A, or GABAA receptors. Our data support a negative allosteric modulation of DRN somatodendritic 5-HT1A receptor by CBD.
Keywords: 5-HT1A receptor; calcium; cannabidiol; dorsal raphe nucleus; firing; rat; slice.
Copyright © 2022 Mendiguren, Aostri, Alberdi, Pérez-Samartín and Pineda.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

