Chrysophanol-8-O-glucoside protects mice against acute liver injury by inhibiting autophagy in hepatic stellate cells and inflammatory response in liver-resident macrophages
- PMID: 36147355
- PMCID: PMC9485814
- DOI: 10.3389/fphar.2022.951521
Chrysophanol-8-O-glucoside protects mice against acute liver injury by inhibiting autophagy in hepatic stellate cells and inflammatory response in liver-resident macrophages
Abstract
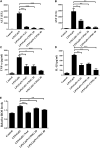
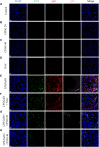
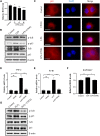
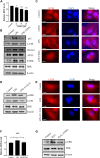
Acute liver failure (ALF) is an unfavorable condition characterized by the rapid loss of liver function and high mortality. Chrysophanol-8-O-glucoside (CPOG) is an anthraquinone derivative isolated from rhubarb. This study aims to evaluate the protective effect of CPOG on lipopolysaccharide (LPS)/D-GalN-induced ALF and its underlying mechanisms. LPS/D-GalN-induced mice ALF model and LPS treatment model in RAW 264.7 and LX2 cells were established. It was found that CPOG ameliorated LPS/D-GalN-induced liver injury and improved mortality as indicated by Hematoxylin-eosin (H&E) staining. Molecularly, qPCR and ELISA results showed that CPOG alleviated LPS/D-GalN-induced release of alanine aminotransferase and aspartate transaminase and the secretion of TNF-α and IL-1β in vivo. LPS/D-GalN-induced intracellular ROS production was also attenuated by CPOG in liver tissue. Further, CPOG attenuated ROS generation and inhibited the expression of p-IκB and p-p65 as well as the expression of TNF-α and IL-1β stimulated by LPS in RAW 264.7 cells. In addition, CPOG alleviated LPS-induced up-regulation of LC3B, p62, ATG5 and Beclin1 by attenuating ROS production and inhibiting MAPK signaling in LX2 cells. Taken together, our data indicated that the CPOG protected against LPS/D-GalN-induced ALF by inhibiting oxidative stress, inflammation response and autophagy. These findings suggest that CPOG could be potential drug for the treatment of ALF in clinic.
Keywords: acute liver injury; autophagy; chrysophanol-8-O-glucoside; lipopolysaccharide; oxidative stress.
Copyright © 2022 Wang, Lu, Qu, Xiong, Wu, Luo, Zhang, Han and Xie.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Protective Role of 4-Octyl Itaconate in Murine LPS/D-GalN-Induced Acute Liver Failure via Inhibiting Inflammation, Oxidative Stress, and Apoptosis.Oxid Med Cell Longev. 2021 Aug 17;2021:9932099. doi: 10.1155/2021/9932099. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34457120 Free PMC article.
-
UMSCs Attenuate LPS/D-GalN-induced Acute Liver Failure in Mice by Down-regulating the MyD88/NF-κB Pathway.J Clin Transl Hepatol. 2021 Oct 28;9(5):690-701. doi: 10.14218/JCTH.2020.00157. Epub 2021 Apr 22. J Clin Transl Hepatol. 2021. PMID: 34722184 Free PMC article.
-
Qingchangligan formula attenuates the inflammatory response to protect the liver from acute failure induced by d-galactosamine/lipopolysaccharide in mice.J Ethnopharmacol. 2017 Apr 6;201:108-116. doi: 10.1016/j.jep.2016.11.007. Epub 2016 Nov 7. J Ethnopharmacol. 2017. PMID: 27833028
-
Salvage effect of E5564, Toll-like receptor 4 antagonist on d-galactosamine and lipopolysaccharide-induced acute liver failure in rats.J Gastroenterol Hepatol. 2010 May;25(5):1009-12. doi: 10.1111/j.1440-1746.2009.06145.x. J Gastroenterol Hepatol. 2010. PMID: 20546456
-
AMPK activation ameliorates D-GalN/LPS-induced acute liver failure by upregulating Foxo3A to induce autophagy.Exp Cell Res. 2017 Sep 15;358(2):335-342. doi: 10.1016/j.yexcr.2017.07.008. Epub 2017 Jul 6. Exp Cell Res. 2017. PMID: 28689811
Cited by
-
Twelve Shugan Lidan Granules from traditional Chinese medicine can improve liver function in patients with postoperative hepatolithiasis by inhibiting the Hippo signaling pathway.Am J Transl Res. 2024 Nov 25;16(11):7186-7199. doi: 10.62347/VXHU6738. eCollection 2024. Am J Transl Res. 2024. PMID: 39678602 Free PMC article.
References
-
- Deng J. K., Zhang X., Wu H. L., Gan Y., Ye L., Zheng H., et al. (2021). ROS-ERK pathway as dual mediators of cellular injury and autophagy-associated adaptive response in urinary protein-irritated renal tubular epithelial cells. J. Diabetes Res. 2021, 6614848. 10.1155/2021/6614848 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

