A study on periconceptional folic acid supplement intake and serum folic acid levels in pregnant mothers
- PMID: 36147391
- PMCID: PMC9485861
- DOI: 10.1016/j.mjafi.2021.05.013
A study on periconceptional folic acid supplement intake and serum folic acid levels in pregnant mothers
Abstract
Background: Survey-based studies have examined the timing of receiving periconceptional folic acid supplementation. To assess the impact of the periconceptional folic acid supplementation, a postulate that multigravida mothers are more likely to have received the supplementation and the level of serum folic acid in them assayed during the first trimester is likely to be higher than primigravida mothers was put forth. Serum folic acid levels were measured in primigravida and multigravida mothers during the first trimester.
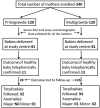
Methods: One hundred twenty primigravida and multigravida mothers registered at antenatal clinic of a tertiary care referral centre were included. Serum folic acid assay from samples collected during the first trimester was carried out by chemiluminescence immuneassay. The mothers were followed up during subsequent OPD visits, during admission for delivery and through mobile phones for assessing the delivery outcomes. World Health Organization cutoff values for serum folic acid were used to analyse the results.
Results: None of the mothers received folic acid supplement before conception. Mean interval from last menstrual period to receiving the first dose of folic acid supplementation was 71.2 days in primigravida and 67.6 days in multigravida mothers. Overall, 21/120 (17.5%) of primigravida mothers and 34/120 (28.3%) of multigravida mothers had serum folic acid values less than 6 ng/ml (deficiency and possible deficiency).
Conclusion: None of the mothers received folic acid supplements before conception. Significant proportion of mothers, particularly the multigravida having less than normal levels serum folic acid indicates correctable lacunae amenable for preventive intervention.
Keywords: Folic acid; Multigravida; Periconceptional; Pregnancy; Primigravida.
© 2021 Director General, Armed Forces Medical Services. Published by Elsevier, a division of RELX India Pvt. Ltd.
Conflict of interest statement
The authors have none to declare.
References
-
- Gupta H., Gupta P. Neural tube defects and folic acid. Indian Pediatr. 2004;41:577–586. - PubMed
-
- Mulinare J., Cordero J.F., Erickson J.D., et al. Periconceptional use of multivitamins and the occurrence of neural tube defects. J Am Med Assoc. 1988;260:3141–3145. - PubMed
-
- Bower C., Stanley F.J. Dietary folate as a risk factor for neural-tube defects: evidence from a case–control study in Western Australia. Med J Aust. 1989;150:613–619. - PubMed
-
- Werler M.M., Shapiro S., Mitchell A.A. Periconceptional folic acid exposure and risk of concurrent neural tube defects. J Am Med Assoc. 1993;269:1257–1261. - PubMed
LinkOut - more resources
Full Text Sources