Modified Qing' e Pills exerts anti-osteoporosis effects and prevents bone loss by enhancing type H blood vessel formation
- PMID: 36147560
- PMCID: PMC9485463
- DOI: 10.3389/fendo.2022.998971
Modified Qing' e Pills exerts anti-osteoporosis effects and prevents bone loss by enhancing type H blood vessel formation
Abstract
Objective: To explore whether the modified Qing' e Pills (MQEP) exerts anti-osteoporotic effects and prevents bone loss by enhancing angiogenesis.
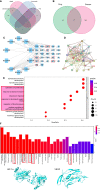
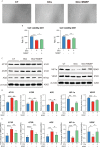
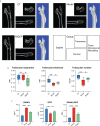
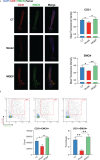
Methods: Network pharmacology was used to assess whether MQEP has a pro-angiogenic capacity and to predict its potential targets. Human umbilical vein endothelial cells were treated with glucocorticoids and MQEP to assess cell viability. The expression of angiotensin II type 1 receptor, angiotensin II type 2 receptor, and angiotensin converting enzyme, which are associated with the activation of the renin-angiotensin-aldosterone system, and the expression of vascular endothelial growth factor and hypoxia-inducible factor 1 alpha, which are associated with the formation of type H blood vessels, were examined by western blot and RT-qPCR. Thereafter, the glucocorticoid-induced osteoporosis model was established and intervened with MQEP. Femur scanning was performed with micro-computed tomography; trabecular spacing, trabecular thickness, and trabecular number were observed and calculated; the expression of nuclear factor-kappa B ligand and osteoprotegerin was detected by ELISA, and the ratio was calculated to evaluate the degree of bone resorption. Finally, type H blood vessels that were highly coupled to osteogenic cells were identified by immunohistochemistry staining and flow cytometry.
Results: This is the first study to reveal and confirm that MQEP could prevent bone loss in glucocorticoid-induced osteoporosis by promoting the expression of hypoxia-inducible factor 1 alpha and vascular endothelial growth factor, which are highly associated with type H blood vessel formation. In vitro experiments confirmed that MQEP could effectively promote the proliferation of vascular endothelial cells and alleviate glucocorticoids-induced activation of the renin-angiotensin-aldosterone system, thereby reducing vascular injury.
Conclusion: MQEP exerts anti-osteoporosis effects and prevents bone loss by alleviating vascular injury caused by renin-angiotensin-aldosterone system activation and promoting type H blood vessel formation.
Keywords: alternative medicine; angiotensin; bone loss; osteoporosis; type H blood vessels; vessel formation.
Copyright © 2022 Lu, Hu, Ma, Xu, Shen, Rong, Zhao and Shuai.
Conflict of interest statement
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Bu-Shen-Tong-Luo decoction prevents bone loss via inhibition of bone resorption and enhancement of angiogenesis in ovariectomy-induced osteoporosis of rats.J Ethnopharmacol. 2018 Jun 28;220:228-238. doi: 10.1016/j.jep.2018.01.007. Epub 2018 Jan 6. J Ethnopharmacol. 2018. PMID: 29317302
-
Glucocorticoids activate the local renin-angiotensin system in bone: possible mechanism for glucocorticoid-induced osteoporosis.Endocrine. 2014 Nov;47(2):598-608. doi: 10.1007/s12020-014-0196-z. Epub 2014 Feb 12. Endocrine. 2014. PMID: 24519760
-
Losartan protects against intermittent hypoxia-induced peritubular capillary loss by modulating the renal renin-angiotensin system and angiogenesis factors.Acta Biochim Biophys Sin (Shanghai). 2020 Jan 2;52(1):38-48. doi: 10.1093/abbs/gmz136. Acta Biochim Biophys Sin (Shanghai). 2020. PMID: 31836883
-
Role of the renin-angiotensin-aldosterone system in bone metabolism.J Bone Miner Metab. 2020 Nov;38(6):772-779. doi: 10.1007/s00774-020-01132-y. Epub 2020 Jul 30. J Bone Miner Metab. 2020. PMID: 32734523 Review.
-
The Relationship between Renin-Angiotensin-Aldosterone System (RAAS) Activity, Osteoporosis and Estrogen Deficiency in Type 2 Diabetes.Int J Mol Sci. 2023 Jul 26;24(15):11963. doi: 10.3390/ijms241511963. Int J Mol Sci. 2023. PMID: 37569338 Free PMC article. Review.
Cited by
-
The influence of modified Qing E Formula on the differential expression of serum exosomal miRNAs in postmenopausal osteoporosis patients.Front Pharmacol. 2024 Sep 4;15:1467298. doi: 10.3389/fphar.2024.1467298. eCollection 2024. Front Pharmacol. 2024. PMID: 39295926 Free PMC article.
-
Osteoporotic osseointegration: therapeutic hallmarks and engineering strategies.Theranostics. 2024 Jun 17;14(10):3859-3899. doi: 10.7150/thno.96516. eCollection 2024. Theranostics. 2024. PMID: 38994021 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

