Changes in Quality of Sleep, Mood, and Other Neuropsychiatric Symptoms After Switching Dolutegravir/Lamivudine/Abacavir to Darunavir/Cobicistat/Emtricitabine/Tenofovir Alafenamide in a Randomized Study of People With Human Immunodeficiency Virus With Poor Sleep Quality: GESIDA 10418
- PMID: 36147597
- PMCID: PMC9487706
- DOI: 10.1093/ofid/ofac345
Changes in Quality of Sleep, Mood, and Other Neuropsychiatric Symptoms After Switching Dolutegravir/Lamivudine/Abacavir to Darunavir/Cobicistat/Emtricitabine/Tenofovir Alafenamide in a Randomized Study of People With Human Immunodeficiency Virus With Poor Sleep Quality: GESIDA 10418
Abstract
Background: Although switching antiretroviral therapy (ART) in people with human immunodeficiency virus experiencing insomnia due to dolutegravir-related neurotoxicity is well founded upon evidence, there is a lack of proof in regard to the outcome of stopping dolutegravir-based ART in people without insomnia but reporting poor sleep quality.
Methods: This is a randomized, multicenter, open-label study to evaluate the reversibility of patient-reported sleep disturbances in patients on dolutegravir/lamivudine/abacavir without insomnia after switching to darunavir/cobicistat/emtricitabine/tenofovir alafenamide. The participants were randomized to switch ART at baseline or at week 4 and then completed 8 weeks of darunavir/cobicistat/emtricitabine/tenofovir alafenamide. Our primary objective was to compare changes in sleep quality between arms at week 4. Secondary objectives were to compare changes in mood and neuropsychiatric symptoms (NS) at week 4 and 4 and 8 weeks after switching to darunavir/cobicistat/emtricitabine/tenofovir alafenamide. The participants completed a survey, including the Pittsburgh Sleep Quality Index (PSQI), the Hospital Anxiety and Depression scale (HADS), and specific questions to explore NS, at each visit to assess those objectives.
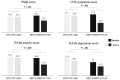
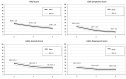
Results: We included 72 participants. The results show that study arms were similar at baseline; however, at week 4, PSQI scores remained unchanged with dolutegravir/lamivudine/abacavir, whereas patients improved significantly after switching to darunavir/cobicistat/emtricitabine/tenofovir alafenamide. Similar differences between arms were also observed in HADS and NS changes. At weeks 4 and 8 after all participants switched to darunavir/cobicistat/emtricitabine/tenofovir alafenamide, we have observed significant improvements in PSQI and HAD scores and in NS.
Conclusions: In patients reporting subclinical sleep disturbances without insomnia, switching from dolutegravir/lamivudine/abacavir to darunavir/cobicistat/emtricitabine/tenofovir alafenamide was associated with better sleep quality and improvements in mood and NS.
Keywords: CNS; clinical trial; darunavir; dolutegravir; neurotoxicity.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures