Young plasma reverses anesthesia and surgery-induced cognitive impairment in aged rats by modulating hippocampal synaptic plasticity
- PMID: 36147703
- PMCID: PMC9485610
- DOI: 10.3389/fnagi.2022.996223
Young plasma reverses anesthesia and surgery-induced cognitive impairment in aged rats by modulating hippocampal synaptic plasticity
Retraction in
-
Retraction: Young plasma reverses anesthesia and surgery-induced cognitive impairment in aged rats by modulating hippocampal synaptic plasticity.Front Aging Neurosci. 2023 Dec 15;15:1349869. doi: 10.3389/fnagi.2023.1349869. eCollection 2023. Front Aging Neurosci. 2023. PMID: 38161588 Free PMC article.
Abstract
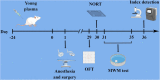
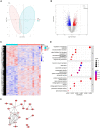
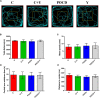
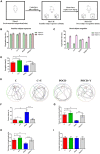
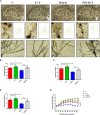
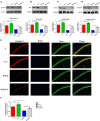
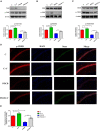
We investigated the protective effect of young plasma on anesthesia- and surgery-induced cognitive impairment and the potential underlying mechanism using bioinformatics, functional enrichment analysis, gene set enrichment analysis, Golgi-Cox staining, dendritic spine analysis, immunofluorescence assay, western blot analysis, and transmission electron microscopy. Furthermore, we performed behavioral assessments using the open field test, the novel object recognition test, and the Morris water maze test. We identified 1969 differentially expressed genes induced by young plasma treatment, including 800 upregulated genes and 1169 downregulated genes, highlighting several enriched biological processes (signal release from synapse, postsynaptic density and neuron to neuron synapse). Anesthesia- and surgery-induced cognitive impairment in aged rats was comparatively less severe following young plasma preinfusion. In addition, the decreased levels of synapse-related and tyrosine kinase B/extracellular signal-regulated protein kinase/cyclic adenosine monophosphate response element-binding protein (TrkB/ERK/CREB) signaling pathway-related proteins, dendritic and spine deficits, and ultrastructural changes were ameliorated in aged mice following young plasma preinfusion. Together, these findings suggest that young plasma reverses anesthesia- and surgery-induced cognitive impairment in aged rats and that the mechanism is associated with the activation of the TrkB/ERK/CREB signaling pathway and improvement in hippocampal synaptic plasticity.
Keywords: TrkB/ERK/CREB signaling pathway; aged; cognitive impairment; synaptic plasticity; young plasma.
Copyright © 2022 Li, Zhang, Yan, Wang, Yu, Yin, Zhou, Hou and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Electroacupuncture pretreatment protects against anesthesia/surgery-induced cognitive decline by activating CREB via the ERK/MAPK pathway in the hippocampal CA1 region in aged rats.Aging (Albany NY). 2023 Oct 19;15(20):11227-11243. doi: 10.18632/aging.205124. Epub 2023 Oct 19. Aging (Albany NY). 2023. PMID: 37857016 Free PMC article.
-
[Electroacupuncture improves learning and memory impairment and enhances hippocampal synaptic plasticity through BDNF/TRKB/CREB signaling pathway in cerebral ischemia-reperfusion injury rats].Zhen Ci Yan Jiu. 2023 Sep 25;48(9):843-51. doi: 10.13702/j.1000-0607.20220742. Zhen Ci Yan Jiu. 2023. PMID: 37730254 Chinese.
-
Electroacupuncture promotes synaptic plasticity in rats with chronic inflammatory pain-related depression by upregulating BDNF/TrkB/CREB signaling pathway.Brain Behav. 2023 Dec;13(12):e3310. doi: 10.1002/brb3.3310. Epub 2023 Nov 10. Brain Behav. 2023. PMID: 37948105 Free PMC article.
-
Long-term neurocognitive dysfunction in offspring via NGF/ ERK/CREB signaling pathway caused by ketamine exposure during the second trimester of pregnancy in rats.Oncotarget. 2017 May 9;8(19):30956-30970. doi: 10.18632/oncotarget.16042. Oncotarget. 2017. PMID: 28415680 Free PMC article.
-
BDNF-induced local protein synthesis and synaptic plasticity.Neuropharmacology. 2014 Jan;76 Pt C:639-56. doi: 10.1016/j.neuropharm.2013.04.005. Epub 2013 Apr 16. Neuropharmacology. 2014. PMID: 23602987 Review.
Cited by
-
Esketamine Inhibits Cocaine-Seeking Behaviour Subsequent to Various Abstinence Conditions in Rats.Biomolecules. 2023 Sep 19;13(9):1411. doi: 10.3390/biom13091411. Biomolecules. 2023. PMID: 37759811 Free PMC article.
-
Polygalae Radix Attenuates Methamphetamine-Induced Behavioral Sensitization Through the TrkB/ERK Pathway in the Caudate Putamen of Mice.Neurochem Res. 2025 Mar 17;50(2):120. doi: 10.1007/s11064-025-04368-0. Neurochem Res. 2025. PMID: 40095175
-
Electroacupuncture pretreatment protects against anesthesia/surgery-induced cognitive decline by activating CREB via the ERK/MAPK pathway in the hippocampal CA1 region in aged rats.Aging (Albany NY). 2023 Oct 19;15(20):11227-11243. doi: 10.18632/aging.205124. Epub 2023 Oct 19. Aging (Albany NY). 2023. PMID: 37857016 Free PMC article.
-
Aging and age-related diseases with a focus on therapeutic potentials of young blood/plasma.Naunyn Schmiedebergs Arch Pharmacol. 2024 Jan;397(1):1-13. doi: 10.1007/s00210-023-02657-5. Epub 2023 Aug 8. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 37552316 Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

