BNT162b2 vaccine effectiveness in chronic kidney disease patients-an observational study
- PMID: 36147707
- PMCID: PMC9384353
- DOI: 10.1093/ckj/sfac166
BNT162b2 vaccine effectiveness in chronic kidney disease patients-an observational study
Abstract
Background: Chronic kidney disease (CKD) is a risk factor for severe coronavirus disease 2019 (COVID-19). We aimed to evaluate the real-life effectiveness of the BNT162b2 messenger RNA vaccine for a range of outcomes in patients with CKD compared with matched controls.
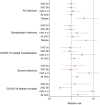
Methods: Data from Israel's largest healthcare organization were retrospectively used. Vaccinated CKD [estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2] and maintenance dialysis patients were matched to vaccinated controls without CKD (eGFR ≥60 ml/min/1.73 m2) according to demographic and clinical characteristics. Study outcomes included documented infection with severe acute respiratory syndrome coronavirus 2, symptomatic infection, COVID-19-related hospitalization, severe disease and death. Vaccine effectiveness was estimated as the risk ratio (RR) at days 7-28 following the second vaccine dose, using the Kaplan-Meier estimator. Effectiveness measures were also evaluated separately for various stages of CKD.
Results: There were 67 861 CKD patients not treated with dialysis, 2606 hemodialysis (HD) patients and 70 467 matched controls. The risk of severe disease {RR 1.84 [95% confidence interval (CI) 0.95-2.67]} and death [RR 2.00 (95% CI 0.99-5.20)] was increased in nondialysis CKD patients compared with controls without CKD following vaccination. For the subgroup of patients with eGFR <30 ml/min/1.73 m2, the risk of severe disease and death was increased compared with controls [RR 6.42 (95% CI 1.85-17.51) and RR 8.81 (95% CI 1.63-13.81), respectively]. The risks for all study outcomes were increased in HD patients compared with controls.
Conclusion: Two doses of the BNT162b2 vaccine were found to be less efficient for patients with eGFR <30 ml/min/1.73 m2. Risk in HD patients is increased for all outcomes. These results suggest prioritizing patients with eGFR <30 ml/min/1.73 m2 for booster shots, pre- and post-exposure prophylaxis and early COVID-19 therapy.
Keywords: BNT162b2 vaccine; COVID-19; chronic kidney disease.
© The Author(s) 2022. Published by Oxford University Press on behalf of the ERA.
Figures




References
-
- World Health Organization. WHO Coronavirus (COVID-19) dashboard . https://covid19.who.int/ (30 December 2021, date last accessed).
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

