Synaptic determinants of cholinergic interneurons hyperactivity during parkinsonism
- PMID: 36147730
- PMCID: PMC9485566
- DOI: 10.3389/fnsyn.2022.945816
Synaptic determinants of cholinergic interneurons hyperactivity during parkinsonism
Abstract
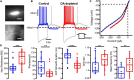
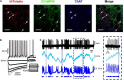
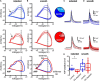
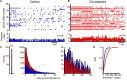
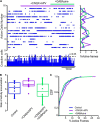
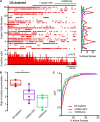
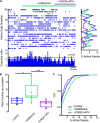
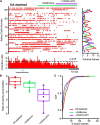
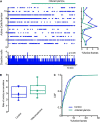
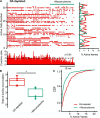
Parkinson's disease is a neurodegenerative ailment generated by the loss of dopamine in the basal ganglia, mainly in the striatum. The disease courses with increased striatal levels of acetylcholine, disrupting the balance among these modulatory transmitters. These modifications disturb the excitatory and inhibitory balance in the striatal circuitry, as reflected in the activity of projection striatal neurons. In addition, changes in the firing pattern of striatal tonically active interneurons during the disease, including cholinergic interneurons (CINs), are being searched. Dopamine-depleted striatal circuits exhibit pathological hyperactivity as compared to controls. One aim of this study was to show how striatal CINs contribute to this hyperactivity. A second aim was to show the contribution of extrinsic synaptic inputs to striatal CINs hyperactivity. Electrophysiological and calcium imaging recordings in Cre-mice allowed us to evaluate the activity of dozens of identified CINs with single-cell resolution in ex vivo brain slices. CINs show hyperactivity with bursts and silences in the dopamine-depleted striatum. We confirmed that the intrinsic differences between the activity of control and dopamine-depleted CINs are one source of their hyperactivity. We also show that a great part of this hyperactivity and firing pattern change is a product of extrinsic synaptic inputs, targeting CINs. Both glutamatergic and GABAergic inputs are essential to sustain hyperactivity. In addition, cholinergic transmission through nicotinic receptors also participates, suggesting that the joint activity of CINs drives the phenomenon; since striatal CINs express nicotinic receptors, not expressed in striatal projection neurons. Therefore, CINs hyperactivity is the result of changes in intrinsic properties and excitatory and inhibitory inputs, in addition to the modification of local circuitry due to cholinergic nicotinic transmission. We conclude that CINs are the main drivers of the pathological hyperactivity present in the striatum that is depleted of dopamine, and this is, in part, a result of extrinsic synaptic inputs. These results show that CINs may be a main therapeutic target to treat Parkinson's disease by intervening in their synaptic inputs.
Keywords: Parkinson’s disease; calcium imaging; cholinergic interneurons; electrophysiology; striatal microcircuit.
Copyright © 2022 Padilla-Orozco, Duhne, Fuentes-Serrano, Ortega, Galarraga, Bargas and Lara-González.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Striatal cholinergic transmission. Focus on nicotinic receptors' influence in striatal circuits.Eur J Neurosci. 2021 Apr;53(8):2421-2442. doi: 10.1111/ejn.15135. Epub 2021 Apr 26. Eur J Neurosci. 2021. PMID: 33529401 Free PMC article. Review.
-
Increased GABAergic transmission in neuropeptide Y-expressing neurons in the dopamine-depleted murine striatum.J Neurophysiol. 2020 Apr 1;123(4):1496-1503. doi: 10.1152/jn.00059.2020. Epub 2020 Mar 11. J Neurophysiol. 2020. PMID: 32159408
-
Striatal cholinergic interneurons display activity-related phosphorylation of ribosomal protein S6.PLoS One. 2012;7(12):e53195. doi: 10.1371/journal.pone.0053195. Epub 2012 Dec 28. PLoS One. 2012. PMID: 23285266 Free PMC article.
-
A tonic nicotinic brake controls spike timing in striatal spiny projection neurons.Elife. 2022 May 17;11:e75829. doi: 10.7554/eLife.75829. Elife. 2022. PMID: 35579422 Free PMC article.
-
Cholinergic/glutamatergic co-transmission in striatal cholinergic interneurons: new mechanisms regulating striatal computation.J Neurochem. 2017 Aug;142 Suppl 2:90-102. doi: 10.1111/jnc.14003. Epub 2017 Apr 18. J Neurochem. 2017. PMID: 28421605 Review.
Cited by
-
Enhancing striatal acetylcholine facilitates dopamine release and striatal output in parkinsonian mice.Cell Biosci. 2024 Dec 3;14(1):146. doi: 10.1186/s13578-024-01328-z. Cell Biosci. 2024. PMID: 39627827 Free PMC article.
-
Rethinking the network determinants of motor disability in Parkinson's disease.Front Synaptic Neurosci. 2023 Jun 28;15:1186484. doi: 10.3389/fnsyn.2023.1186484. eCollection 2023. Front Synaptic Neurosci. 2023. PMID: 37448451 Free PMC article. Review.
-
Distributed dopaminergic signaling in the basal ganglia and its relationship to motor disability in Parkinson's disease.Curr Opin Neurobiol. 2023 Dec;83:102798. doi: 10.1016/j.conb.2023.102798. Epub 2023 Oct 30. Curr Opin Neurobiol. 2023. PMID: 37866012 Free PMC article. Review.
-
The dual role of striatal interneurons: circuit modulation and trophic support for the basal ganglia.Neural Regen Res. 2024 Jun 1;19(6):1277-1283. doi: 10.4103/1673-5374.382987. Epub 2023 Aug 14. Neural Regen Res. 2024. PMID: 37905876 Free PMC article.
-
Insights into Calpain Activation and Rho-ROCK Signaling in Parkinson's Disease and Aging.Biomedicines. 2024 May 13;12(5):1074. doi: 10.3390/biomedicines12051074. Biomedicines. 2024. PMID: 38791036 Free PMC article. Review.
References
-
- Abbondanza A., Ribeiro Bas I., Modrak M., Capek M., Minich J., Tyshkevich A., et al. (2022). Nicotinic acetylcholine receptors expressed by striatal interneurons inhibit striatal activity and control striatal-dependent behaviors. J. Neurosci. 42 2786–2803. 10.1523/jneurosci.1627-21.2022 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

