Regulation and coordination of the different DNA damage responses in Drosophila
- PMID: 36147740
- PMCID: PMC9486394
- DOI: 10.3389/fcell.2022.993257
Regulation and coordination of the different DNA damage responses in Drosophila
Abstract
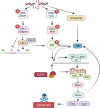
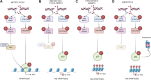
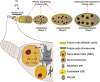
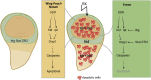
Cells have evolved mechanisms that allow them to respond to DNA damage to preserve genomic integrity and maintain tissue homeostasis. These responses include the activation of the cell cycle checkpoints and the repair mechanisms or the induction of apoptosis that eventually will eliminate damaged cells. These "life" vs. "death" decisions differ depending on the cell type, stages of development, and the proliferation status of the cell. The apoptotic response after DNA damage is of special interest as defects in its induction could contribute to tumorigenesis or the resistance of cancer cells to therapeutic agents such as radiotherapy. Multiples studies have elucidated the molecular mechanisms that mediate the activation of the DNA damage response pathway (DDR) and specifically the role of p53. However, much less is known about how the different cellular responses such as cell proliferation control and apoptosis are coordinated to maintain tissue homeostasis. Another interesting question is how the differential apoptotic response to DNA damage is regulated in distinct cell types. The use of Drosophila melanogaster as a model organism has been fundamental to understand the molecular and cellular mechanisms triggered by genotoxic stress. Here, we review the current knowledge regarding the cellular responses to ionizing radiation as the cause of DNA damage with special attention to apoptosis in Drosophila: how these responses are regulated and coordinated in different cellular contexts and in different tissues. The existence of intrinsic mechanisms that might attenuate the apoptotic pathway in response to this sort of DNA damage may well be informative for the differences in the clinical responsiveness of tumor cells after radiation therapy.
Keywords: DNA damage response; Drosophila; apoptosis; cell cycle; cellular context; ionizing radiation; p53; tissue homeostasis.
Copyright © 2022 Baonza, Tur-Gracia, Pérez-Aguilera and Estella.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

