Revealing the novel effect of Jinghua Weikang capsule against the antibiotic resistance of Helicobacter pylori
- PMID: 36147839
- PMCID: PMC9485998
- DOI: 10.3389/fmicb.2022.962354
Revealing the novel effect of Jinghua Weikang capsule against the antibiotic resistance of Helicobacter pylori
Abstract
Background: Helicobacter pylori (H. pylori) infects half of the human population globally. Eradication rates with triple or quadruple therapy have decreased owing to the increasing rate of antibiotic resistance. Jinghua Weikang capsule (JWC) is the first and most popular Chinese patent medicine approved by the state for the treatment of gastritis and peptic ulcers caused by H. pylori infection in China. Previous studies have found that JWC has a certain bactericidal effect on drug-resistant H. pylori and its major component, Chenopodium ambrosioides L. inhibits biofilm formation, but the mechanism remains unclear. This study focused on drug-resistant H. pylori and explored whether JWC could reverse drug resistance and its related mechanisms.
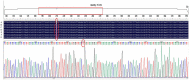
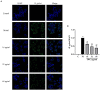
Method: The agar plate dilution method, E-test method, and killing kinetics assay were used to evaluate the bactericidal effect of JWC on antibiotic-resistant H. pylori and its effect on antibiotic resistance. Sanger sequencing was used to detect mutations in drug resistance genes. The crystal violet method, scanning electron microscopy, and confocal laser scanning microscopy were used to evaluate the effects of JWC on biofilms. qPCR was performed to evaluate the effect of JWC on the expression of efflux pump-related genes. qPCR and immunofluorescence were used to evaluate the effects of JWC on H. pylori adhesion.
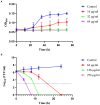
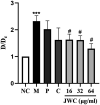
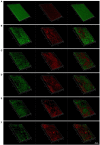
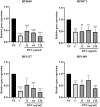
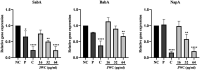
Results: JWC showed considerable antibacterial activity against drug-resistant H. pylori strains, with minimum inhibitory concentration (MIC) values ranging from 64 to 1,024 μg/ml. The MIC of metronidazole (MTZ) against H. pylori 26,695-16R decreased from 64 to 6 μg/ml after treatment with 1/2 MIC of JWC. The resistance of H. pylori 26,695-16R to MTZ was reversed by JWC, and its effect was better than that of PaβN and CCCP. H. pylori 26,695-16R is a moderate biofilm-forming strain, and JWC (16-64 μg/ml) can inhibit the formation of biofilms in H. pylori 26,695-16R. JWC reduced the expression of HP0605-HP0607 (hefABC), HP0971-HP0969 (hefDEF), HP1327-HP1329 (hefGHI), and HP1489-HP1487. JWC reduced the adhesion of H. pylori to GES-1 cells and the expression of adhesives NapA, SabA, and BabA.
Conclusion: The reversal of MTZ resistance by JWC may be achieved through the adhesin/efflux pump-biofilm pathway.
Keywords: Helicobacter pylori; Jinghua Weikang capsule; adhesion; antibiotic resistance; biofilm; efflux pump; metronidazole.
Copyright © 2022 Jia, Huang, Lin, Chu, Shi, Zhang and Ye.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Effect and mechanism of Qingre Huashi decoction on drug-resistant Helicobacter pylori.World J Gastroenterol. 2024 Jun 28;30(24):3086-3105. doi: 10.3748/wjg.v30.i24.3086. World J Gastroenterol. 2024. PMID: 38983958 Free PMC article.
-
In vitro bactericidal activity of Jinghua Weikang Capsule and its individual herb Chenopodium ambrosioides L. against antibiotic-resistant Helicobacter pylori.Chin J Integr Med. 2013 Jan;19(1):54-7. doi: 10.1007/s11655-012-1248-y. Epub 2012 Dec 29. Chin J Integr Med. 2013. PMID: 23275015
-
Efficacy and safety of Jinghua Weikang capsule combined with amoxicillin-furazolidone triple/quadruple therapies in the rescue treatment of Helicobacter pylori infection.Front Med (Lausanne). 2025 Mar 25;12:1531620. doi: 10.3389/fmed.2025.1531620. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40201322 Free PMC article.
-
Jinghua Weikang capsule for helicobacter pylori eradication: A systematic review and meta-analysis with trial sequential analysis.Front Pharmacol. 2022 Sep 26;13:959184. doi: 10.3389/fphar.2022.959184. eCollection 2022. Front Pharmacol. 2022. PMID: 36225593 Free PMC article.
-
Novel and Effective Therapeutic Regimens for Helicobacter pylori in an Era of Increasing Antibiotic Resistance.Front Cell Infect Microbiol. 2017 May 5;7:168. doi: 10.3389/fcimb.2017.00168. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28529929 Free PMC article. Review.
Cited by
-
A new approach to overcoming antibiotic-resistant bacteria: Traditional Chinese medicine therapy based on the gut microbiota.Front Cell Infect Microbiol. 2023 Apr 6;13:1119037. doi: 10.3389/fcimb.2023.1119037. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37091671 Free PMC article. Review.
-
Helicobacter pylori Efflux Pumps: A Double-Edged Sword in Antibiotic Resistance and Biofilm Formation.Int J Mol Sci. 2024 Nov 14;25(22):12222. doi: 10.3390/ijms252212222. Int J Mol Sci. 2024. PMID: 39596287 Free PMC article. Review.
-
Based on quorum sensing: reverse effect of traditional Chinese medicine on bacterial drug resistance mechanism.Front Cell Infect Microbiol. 2025 Jun 3;15:1582003. doi: 10.3389/fcimb.2025.1582003. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40568697 Free PMC article. Review.
-
Progress in traditional Chinese medicine against chronic gastritis: From chronic non-atrophic gastritis to gastric precancerous lesions.Heliyon. 2023 May 27;9(6):e16764. doi: 10.1016/j.heliyon.2023.e16764. eCollection 2023 Jun. Heliyon. 2023. PMID: 37313135 Free PMC article. Review.
-
In vitro anti-Helicobacter pylori activity and the underlining mechanism of an empirical herbal formula - Hezi Qingyou.Front Microbiol. 2024 Feb 19;15:1355460. doi: 10.3389/fmicb.2024.1355460. eCollection 2024. Front Microbiol. 2024. PMID: 38440143 Free PMC article.
References
-
- Acio-Pizzarello C. R., Acio A. A., Choi E. J., Bond K., Kim J., Kenan A. C., et al. . (2017). Determinants of the regulation of helicobacter pylori adhesins include repeat sequences in both promoter and coding regions as well as the two-component system ArsRS. J. Med. Microbiol. 66, 798–807. doi: 10.1099/jmm.0.000491, PMID: - DOI - PubMed
-
- Chiang T. H., Chang W. J., Chen S. L., Yen A. M., Fann J. C., Chiu S. Y., et al. . (2021). Mass eradication of helicobacter pylori to reduce gastric cancer incidence and mortality: a long-term cohort study on Matsu Islands. Gut 70, 243–250. doi: 10.1136/gutjnl-2020-322200, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

