Myoferlin disturbs redox equilibrium to accelerate gastric cancer migration
- PMID: 36147922
- PMCID: PMC9486956
- DOI: 10.3389/fonc.2022.905230
Myoferlin disturbs redox equilibrium to accelerate gastric cancer migration
Abstract
Objective: In contrast to normal cells, in which reactive oxygen species (ROS) are maintained in redox equilibrium, cancer cells are characterized by ectopic ROS accumulation. Myoferlin, a newly identified oncogene, has been associated with tumor metastasis, intracellular ROS production, and energy metabolism. The mechanism by which myoferlin regulates gastric cancer cell migration and ROS accumulation has not been determined.
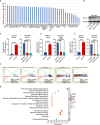
Methods: Myoferlin expression, intracellular ROS levels, the ratios of reduced to oxidized glutathione (GSH/GSSG) and nicotinamide adenine dinucleotide phosphate (NADPH/NADP+) and migratory ability were measured in gastric cancer cells in vitro and in the TCGA and GEO databases in silico.
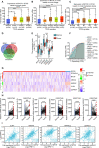
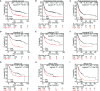
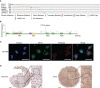
Results: Myoferlin was found to be more highly expressed in tumor than in normal tissues of gastric cancer patients, with higher expression of Myoferlin associated with shorter survival time. Myoferlin was associated with significantly higher intracellular ROS levels and enhanced migration of gastric cancer cells. N-acetyl-L-cysteine (NAC), a potent inhibitor of ROS, inhibited Myoferlin-induced ROS accumulation and cell migration.
Conclusions: Myoferlin is a candidate prognostic biomarker for gastric cancer and plays an essential role in regulating redox equilibrium and gastric cancer cell migration. Myoferlin may also be a new target for treatment of patients with gastric cancer.
Keywords: GSH; ROS; gastric cancer; metastasis; myoferlin.
Copyright © 2022 Shi, Cheng, Shi, Liu, Yang, Wang, Wei, Chen and Fang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Relationship between gluconeogenesis and glutathione redox state in rabbit kidney-cortex tubules.Metabolism. 2003 Jun;52(6):739-46. doi: 10.1016/s0026-0495(03)00035-0. Metabolism. 2003. PMID: 12800101
-
Aloin Inhibits the Proliferation and Migration of Gastric Cancer Cells by Regulating NOX2-ROS-Mediated Pro-Survival Signal Pathways.Drug Des Devel Ther. 2020 Jan 14;14:145-155. doi: 10.2147/DDDT.S219247. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32021099 Free PMC article.
-
A spontaneous mutation in the nicotinamide nucleotide transhydrogenase gene of C57BL/6J mice results in mitochondrial redox abnormalities.Free Radic Biol Med. 2013 Oct;63:446-56. doi: 10.1016/j.freeradbiomed.2013.05.049. Epub 2013 Jun 7. Free Radic Biol Med. 2013. PMID: 23747984
-
The Central Role of Amino Acids in Cancer Redox Homeostasis: Vulnerability Points of the Cancer Redox Code.Front Oncol. 2017 Dec 21;7:319. doi: 10.3389/fonc.2017.00319. eCollection 2017. Front Oncol. 2017. PMID: 29312889 Free PMC article. Review.
-
Myoferlin, a multifunctional protein in normal cells, has novel and key roles in various cancers.J Cell Mol Med. 2019 Nov;23(11):7180-7189. doi: 10.1111/jcmm.14648. Epub 2019 Sep 1. J Cell Mol Med. 2019. PMID: 31475450 Free PMC article. Review.
Cited by
-
Characterization of mRNA Signature in Milk Small Extracellular Vesicles from Cattle Infected with Bovine Leukemia Virus.Pathogens. 2023 Oct 13;12(10):1239. doi: 10.3390/pathogens12101239. Pathogens. 2023. PMID: 37887755 Free PMC article.
-
Phosphate-to-alanine ratio and bilirubin-to-androsterone glucuronide ratio are the hub metabolites in upper gastrointestinal cancers: a Mendelian randomisation (MR) study.Ecancermedicalscience. 2024 Jul 23;18:1731. doi: 10.3332/ecancer.2024.1731. eCollection 2024. Ecancermedicalscience. 2024. PMID: 39421169 Free PMC article. Review.
-
The role of reactive oxygen species in gastric cancer.Cancer Biol Med. 2024 Jul 9;21(9):740-53. doi: 10.20892/j.issn.2095-3941.2024.0182. Cancer Biol Med. 2024. PMID: 38982978 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

