Detection of innate immune response modulating impurities (IIRMI) in therapeutic peptides and proteins: Impact of excipients
- PMID: 36148237
- PMCID: PMC9485840
- DOI: 10.3389/fimmu.2022.970499
Detection of innate immune response modulating impurities (IIRMI) in therapeutic peptides and proteins: Impact of excipients
Abstract
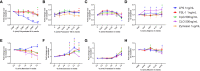
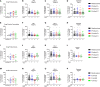
Unintended immunogenicity can affect the safety and efficacy of therapeutic proteins and peptides, so accurate assessments of immunogenicity risk can aid in the selection, development, and regulation of biologics. Product- and process- related impurities can act as adjuvants that activate the local or systemic innate immune response increasing the likelihood of product immunogenicity. Thus, assessing whether products have innate immune response modulating impurities (IIRMI) is a key component of immunogenicity risk assessments. Identifying trace levels of individual IIRMI can be difficult and testing individually for all potential impurities is not feasible. Therefore, to mitigate the risk, cell-based assays that use human blood cells or monocyte-macrophage reporter cell lines are being developed to detect minute quantities of impurities capable of eliciting innate immune activation. As these are cell-based assays, there is concern that excipients could blunt the cell responses, masking the presence of immunogenic IIRMI. Here, we explore the impact of frequently used excipients (non-ionic detergents, sugars, amino acids, bulking agents) on the sensitivity of reporter cell lines (THP-1- and RAW-Blue cells) and fresh human blood cells to detect purified TLR agonists as model IIRMI. We show that while excipients do not modulate the innate immune response elicited by TLR agonists in vivo, they can impact on the sensitivity of cell-based IIRMI assays. Reduced sensitivity to detect LPS, FSL-1, and other model IIRMI was also evident when testing 3 different recombinant drug products, product A (a representative mAb), B (a representative growth factor), C (a representative peptide), and their corresponding formulations. These results indicate that product formulations need to be considered when developing and validating cell-based assays for assessing clinically relevant levels of IIRMI in therapeutic proteins. Optimization of reporter cells, culture conditions and drug product concentration appear to be critical to minimize the impact of excipients and attain sensitive and reproducible assays.
Keywords: IIRMI; In vitro model; excipient; immunogenicity; masking; reporter cell lines.
Copyright © 2022 Thacker, Her, Kelley-Baker, Ireland, Manangeeswaran, Pang and Verthelyi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Cell-Based Assays to Detect Innate Immune Response Modulating Impurities: Application to Biosimilar Insulin.AAPS J. 2024 Dec 20;27(1):20. doi: 10.1208/s12248-024-00983-x. AAPS J. 2024. PMID: 39707070
-
Assessment of innate immune response modulating impurities (IIRMI) in synthetic peptide drugs (liraglutide).Biochem Biophys Res Commun. 2025 Jul 22;771:151967. doi: 10.1016/j.bbrc.2025.151967. Epub 2025 May 13. Biochem Biophys Res Commun. 2025. PMID: 40398094
-
Aggregates of IVIG or Avastin, but not HSA, modify the response to model innate immune response modulating impurities.Sci Rep. 2018 Jul 31;8(1):11477. doi: 10.1038/s41598-018-29850-4. Sci Rep. 2018. PMID: 30065306 Free PMC article.
-
Reactive impurities in excipients: profiling, identification and mitigation of drug-excipient incompatibility.AAPS PharmSciTech. 2011 Dec;12(4):1248-63. doi: 10.1208/s12249-011-9677-z. Epub 2011 Sep 27. AAPS PharmSciTech. 2011. PMID: 21948318 Free PMC article. Review.
-
Excipients in parenteral formulations: selection considerations and effective utilization with small molecules and biologics.Drug Dev Ind Pharm. 2018 Oct;44(10):1565-1571. doi: 10.1080/03639045.2018.1483392. Epub 2018 Jul 13. Drug Dev Ind Pharm. 2018. PMID: 29863908 Review.
Cited by
-
Assessing the immunogenicity risk of salmon calcitonin peptide impurities using in silico and in vitro methods.Front Pharmacol. 2024 Aug 9;15:1363139. doi: 10.3389/fphar.2024.1363139. eCollection 2024. Front Pharmacol. 2024. PMID: 39185315 Free PMC article.
-
Immunogenicity of therapeutic peptide products: bridging the gaps regarding the role of product-related risk factors.Front Immunol. 2025 Jun 18;16:1608401. doi: 10.3389/fimmu.2025.1608401. eCollection 2025. Front Immunol. 2025. PMID: 40607385 Free PMC article. Review.
-
Assessing developability early in the discovery process for novel biologics.MAbs. 2023 Jan-Dec;15(1):2171248. doi: 10.1080/19420862.2023.2171248. MAbs. 2023. PMID: 36823021 Free PMC article. Review.
-
Influence of Nano-Particulate Impurities and β-glucans on the Stability of Protein-Based Formulations.Curr Drug Deliv. 2025;22(6):659-665. doi: 10.2174/0115672018290111240119115306. Curr Drug Deliv. 2025. PMID: 38299274 Review.
-
Cell-Based Assays to Detect Innate Immune Response Modulating Impurities: Application to Biosimilar Insulin.AAPS J. 2024 Dec 20;27(1):20. doi: 10.1208/s12248-024-00983-x. AAPS J. 2024. PMID: 39707070
References
-
- FDA . Guidance for industry: Immunogenicity assessment for therapeutic protein products. In: Administration CfDEaRCCfBEaRCFaD. Silver Spring, Maryland: Food and Drug Administration. (2014).
-
- EMA . Guideline on immunogenicity assessment of therapeutic proteins In: Party BMPW, editor. 01/06/2017 ed. 30 Churchhill Place Conary Wharf London UK: European Medicines Agency; (2017).
-
- Mahlangu JN, Weldingh KN, Lentz SR, Kaicker S, Karim FA, Matsushita T, et al. . Changes in the amino acid sequence of the recombinant human factor VIIa analog, vatreptacog alfa, are associated with clinical immunogenicity. J Thromb haemost.: JTH (2015) 13(11):1989–98. doi: 10.1111/jth.13141 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

