Post-translational dysregulation of glucose uptake during exhaustive cycling exercise in vastus lateralis muscle of healthy homozygous carriers of the ACE deletion allele
- PMID: 36148310
- PMCID: PMC9488703
- DOI: 10.3389/fphys.2022.933792
Post-translational dysregulation of glucose uptake during exhaustive cycling exercise in vastus lateralis muscle of healthy homozygous carriers of the ACE deletion allele
Abstract
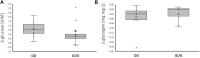
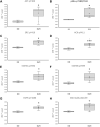
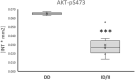
Homozygous carriers of the deletion allele in the gene for angiotensin-converting enzyme (ACE-DD) demonstrate an elevated risk to develop inactivity-related type II diabetes and show an overshoot of blood glucose concentration with enduring exercise compared to insertion allele carriers. We hypothesized that ACE-DD genotypes exhibit a perturbed activity of signaling processes governing capillary-dependent glucose uptake in vastus lateralis muscle during exhaustive cycling exercise, which is associated with the aerobic fitness state. 27 healthy, male white Caucasian subjects (26.8 ± 1.1 years; BMI 23.6 +/- 0.6 kg m-2) were characterized for their aerobic fitness based on a threshold of 50 ml O2 min-1 kg-1 and the ACE-I/D genotype. Subjects completed a session of exhaustive one-legged exercise in the fasted state under concomitant measurement of cardiorespiratory function. Capillary blood and biopsies were collected before, and ½ and 8 h after exercise to quantify glucose and lipid metabolism-related compounds (lipoproteins, total cholesterol, ketones) in blood, the phosphorylation of 45 signaling proteins, muscle glycogen and capillaries. Effects of aerobic fitness, ACE-I/D genotype, and exercise were assessed with analysis of variance (ANOVA) under the hypothesis of a dominant effect of the insertion allele. Exertion with one-legged exercise manifested in a reduction of glycogen concentration ½ h after exercise (-0.046 mg glycogen mg-1 protein). Blood glucose concentration rose immediately after exercise in association with the ACE-I/D genotype (ACE-DD: +26%, ACE-ID/II: +6%) and independent of the fitness state (p = 0.452). Variability in total cholesterol was associated with exercise and fitness. In fit subjects, the phosphorylation levels of glucose uptake-regulating kinases [AKT-pT308 (+156%), SRC-pY419, p38α-pT180/T182, HCK-pY411], as well as cytokine/angiotensin 1-7 signaling factors [(STAT5A-pY694, STAT5B-pY699, FYN-pY420, EGFR-pY1086] were higher in angiotensin converting enzyme I-allele carriers than ACE-DD genotypes after exercise. Conversely, the AKT-S473 phosphorylation level (+117%) and angiotensin 2's blood concentration (+191%) were higher in ACE-DD genotypes. AKT-S473 phosphorylation levels post-exercise correlated to anatomical parameters of muscle performance and metabolic parameters (p < 0.05 and │r│>0.70). The observations identify reciprocal alterations of S473 and T308 phosphorylation of AKT as gatekeeper of a post-translational dysregulation of transcapillary glucose uptake in ACE-DD genotypes which may be targeted in personalized approaches to mitigate type II diabetes.
Keywords: angiotensin; diabetes; exercise; genotype; signalling.
Copyright © 2022 Flück, Vaughan, Rittweger and Giraud.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
ACE-I/D Allele Modulates Improvements of Cardiorespiratory Function and Muscle Performance with Interval-Type Exercise.Genes (Basel). 2023 May 17;14(5):1100. doi: 10.3390/genes14051100. Genes (Basel). 2023. PMID: 37239460 Free PMC article.
-
The Angiotensin Converting Enzyme Insertion/Deletion Polymorphism Modifies Exercise-Induced Muscle Metabolism.PLoS One. 2016 Mar 16;11(3):e0149046. doi: 10.1371/journal.pone.0149046. eCollection 2016. PLoS One. 2016. PMID: 26982073 Free PMC article.
-
Variability in the Aerobic Fitness-Related Dependence on Respiratory Processes During Muscle Work Is Associated With the ACE-I/D Genotype.Front Sports Act Living. 2022 May 19;4:814974. doi: 10.3389/fspor.2022.814974. eCollection 2022. Front Sports Act Living. 2022. PMID: 35663500 Free PMC article.
-
Accelerated Muscle Deoxygenation in Aerobically Fit Subjects During Exhaustive Exercise Is Associated With the ACE Insertion Allele.Front Sports Act Living. 2022 Feb 28;4:814975. doi: 10.3389/fspor.2022.814975. eCollection 2022. Front Sports Act Living. 2022. PMID: 35295536 Free PMC article.
-
The Metabolic Response of Skeletal Muscle to Endurance Exercise Is Modified by the ACE-I/D Gene Polymorphism and Training State.Front Physiol. 2017 Dec 14;8:993. doi: 10.3389/fphys.2017.00993. eCollection 2017. Front Physiol. 2017. PMID: 29311951 Free PMC article.
Cited by
-
ACE-I/D Allele Modulates Improvements of Cardiorespiratory Function and Muscle Performance with Interval-Type Exercise.Genes (Basel). 2023 May 17;14(5):1100. doi: 10.3390/genes14051100. Genes (Basel). 2023. PMID: 37239460 Free PMC article.
-
T-Allele Carriers of Mono Carboxylate Transporter One Gene Polymorphism rs1049434 Demonstrate Altered Substrate Metabolization during Exhaustive Exercise.Genes (Basel). 2024 Jul 14;15(7):918. doi: 10.3390/genes15070918. Genes (Basel). 2024. PMID: 39062697 Free PMC article.
-
On the aspiration to decode the impact of genomics on performance in power and endurance sports.Hum Genomics. 2025 May 16;19(1):55. doi: 10.1186/s40246-025-00750-9. Hum Genomics. 2025. PMID: 40380348 Free PMC article. No abstract available.
-
Association between polymorphisms of the adenylate cyclase 3 gene rs2241759 and the effect of high-intensity interval training on blood lipid profiles.PeerJ. 2025 Apr 11;13:e19271. doi: 10.7717/peerj.19271. eCollection 2025. PeerJ. 2025. PMID: 40231066 Free PMC article.
References
-
- Akhtar S., Yousif M. H. M., Dhaunsi G. S., Chandrasekhar B., Al-Farsi O., Benter I. F. (2012). Angiotensin-(1-7) inhibits epidermal growth factor receptor transactivation via a Mas receptor-dependent pathway. Br. J. Pharmacol. 165 (5), 1390–1400. 10.1111/j.1476-5381.2011.01613.x - DOI - PMC - PubMed
-
- Álvarez C., Ramírez-Campillo R., Ramírez-Vélez R., Izquierdo M. (2017). Effects and prevalence of nonresponders after 12 weeks of high-intensity interval or resistance training in women with insulin resistance: A randomized trial. J. Appl. Physiol. 122 (4), 985–996. 10.1152/japplphysiol.01037.2016 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

