Activation of AMPK-PGC-1α pathway ameliorates peritoneal dialysis related peritoneal fibrosis in mice by enhancing mitochondrial biogenesis
- PMID: 36148521
- PMCID: PMC9518249
- DOI: 10.1080/0886022X.2022.2126789
Activation of AMPK-PGC-1α pathway ameliorates peritoneal dialysis related peritoneal fibrosis in mice by enhancing mitochondrial biogenesis
Abstract
Background: The pathogenesis of peritoneal dialysis (PD)-related peritoneal fibrosis (PF) is not clearly understood, and current treatment options are limited.
Methods: In this study, the effect of PD-related PF on mitochondrial biogenesis was investigated, and the effect of activation of the adenosine monophosphate-activated protein kinase (AMPK)-PGC-1α (peroxisome proliferator-activated receptor γ coactivator-1α) pathway on PF was evaluated in mice.
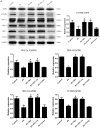
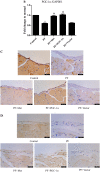
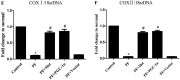
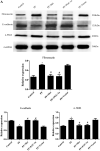
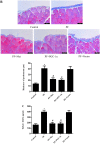
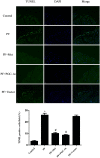
Results: In a mouse model of PD-related PF, AMPK-PGC-1α signaling (phospho-AMPK, PGC-1α, NRF-1, NRF-2 and TFAM expression) was downregulated, mitochondrial DNA (mtDNA) levels were reduced, and mitochondrial structure was damaged in the peritoneum. In addition, TdT-mediated dUTP nick-end labeling (TUNEL) staining showed typical apoptosis characteristics in peritoneal mesothelial cells (PMCs). Activation of the AMPK-PGC-1α pathway (PGC-1α overexpression or metformin, which is an agonist of AMPK) upregulated phospho-AMPK, PGC-1α, nuclear respiratory factors 1 (NRF-1) and 2 (NRF-2), and mitochondrial transcription factor A (TFAM) expression and mtDNA content, improved mitochondrial morphological manifestations, inhibited apoptosis of PMCs and alleviated PF.
Conclusion: Our study may suggest that activation of the AMPK-PGC-1α pathway ameliorates PD-related PF by enhancing mitochondrial biogenesis.
Keywords: AMPK; PGC-1α; Peritoneal dialysis; mitochondrial biogenesis; peritoneal fibrosis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
-
- Li PK, Chow KM, Van de Luijtgaarden MW, et al. . Changes in the worldwide epidemiology of peritoneal dialysis. Nat Rev Nephrol. 2017;13(2):90–103. - PubMed
-
- Davies SJ. Peritoneal dialysis-current status and future challenges. Nat Rev Nephrol. 2013;9(7):399–408. - PubMed
-
- Devuyst O, Margetts PJ, Topley N.. The pathophysiology of the peritoneal membrane. J Am Soc Nephrol. 2010;21(7):1077–1085. - PubMed
-
- Zhou Q, Bajo MA, Del Peso G, et al. . Preventing peritoneal membrane fibrosis in peritoneal dialysis patients. Kidney Int. 2016;90(3):515–524. - PubMed
-
- Krediet RT, Struijk DG.. Peritoneal changes in patients on long-term peritoneal dialysis. Nat Rev Nephrol. 2013;9(7):419–429. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
