Inhibition of YAP/TAZ-driven TEAD activity prevents growth of NF2-null schwannoma and meningioma
- PMID: 36148553
- PMCID: PMC10115179
- DOI: 10.1093/brain/awac342
Inhibition of YAP/TAZ-driven TEAD activity prevents growth of NF2-null schwannoma and meningioma
Erratum in
-
Correction to: Inhibition of YAP/TAZ-driven TEAD activity prevents growth of NF2-null schwannoma and meningioma.Brain. 2025 Mar 6;148(3):e16-e20. doi: 10.1093/brain/awae353. Brain. 2025. PMID: 39576720 Free PMC article. No abstract available.
Abstract
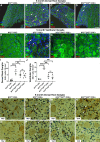
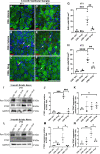
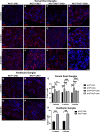
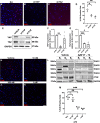
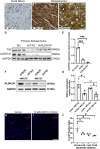
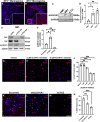
Schwannoma tumours typically arise on the eighth cranial nerve and are mostly caused by loss of the tumour suppressor Merlin (NF2). There are no approved chemotherapies for these tumours and the surgical removal of the tumour carries a high risk of damage to the eighth or other close cranial nerve tissue. New treatments for schwannoma and other NF2-null tumours such as meningioma are urgently required. Using a combination of human primary tumour cells and mouse models of schwannoma, we have examined the role of the Hippo signalling pathway in driving tumour cell growth. Using both genetic ablation of the Hippo effectors YAP and TAZ as well as novel TEAD palmitoylation inhibitors, we show that Hippo signalling may be successfully targeted in vitro and in vivo to both block and, remarkably, regress schwannoma tumour growth. In particular, successful use of TEAD palmitoylation inhibitors in a preclinical mouse model of schwannoma points to their potential future clinical use. We also identify the cancer stem cell marker aldehyde dehydrogenase 1A1 (ALDH1A1) as a Hippo signalling target, driven by the TAZ protein in human and mouse NF2-null schwannoma cells, as well as in NF2-null meningioma cells, and examine the potential future role of this new target in halting schwannoma and meningioma tumour growth.
Keywords: Hippo pathway; Merlin; TEAD proteins; meningioma; schwannoma.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
T.T.T. and L.P. are employees of Vivace Therapeutics and have equity interest in Vivace Therapeutics.
Figures


References
-
- Antinheimo J, Sankila R, Carpen O, Pukkala E, Sainio M, Jaaskelainen J. Population-based analysis of sporadic and type 2 neurofibromatosis-associated meningiomas and schwannomas. Neurology. 2000;54:71–76. - PubMed
-
- Evans DG, Moran A, King A, Saeed S, Gurusinghe N, Ramsden R. Incidence of vestibular schwannoma and neurofibromatosis 2 in the north west of England over a 10-year period: Higher incidence than previously thought. Otol Neurotol. 2005;26:93–97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

