Thermoresponsive and Injectable Hydrogel for Tissue Agnostic Regeneration
- PMID: 36148581
- PMCID: PMC11468498
- DOI: 10.1002/adhm.202201714
Thermoresponsive and Injectable Hydrogel for Tissue Agnostic Regeneration
Abstract
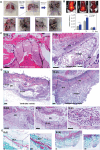
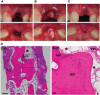
Injectable hydrogels can support the body's innate healing capability by providing a temporary matrix for host cell ingrowth and neovascularization. The clinical adoption of current injectable systems remains low due to their cumbersome preparation requirements, device malfunction, product dislodgment during administration, and uncontrolled biological responses at the treatment site. To address these challenges, a fully synthetic and ready-to-use injectable biomaterial is engineered that forms an adhesive hydrogel that remains at the administration site regardless of defect anatomy. The product elicits a negligible local inflammatory response and fully resorbs into nontoxic components with minimal impact on internal organs. Preclinical animal studies confirm that the engineered hydrogel upregulates the regeneration of both soft and hard tissues by providing a temporary matrix to support host cell ingrowth and neovascularization. In a pilot clinical trial, the engineered hydrogel is successfully administered to a socket site post tooth extraction and forms adhesive hydrogel that stabilizes blood clot and supports soft and hard tissue regeneration. Accordingly, this injectable hydrogel exhibits high therapeutic potential and can be adopted to address multiple unmet needs in different clinical settings.
Keywords: injectable hydrogel; platform technology; regenerative medicine.
© 2022 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
A.F., T.A., S.M., and J.M. are employed by Tetratherix. A.F. and F.D. are co‐inventors in AU2016301103; EP16829490.8; JP2018‐503480; US15/746810; U2016314146; EP344304; JP2018‐529692; US 17/090078; EP 2794701; US9546235. A.F., D.C., and T.A. are co‐inventors in PCT/AU2020/051332 and Chinese national phase patent application (2020800006185.0). D.C. is a clinical advisor to Tetratherix. While the material in the current study has not been published, the main authors have previously invented and disclosed information pertaining to a family of polymers as outlined in granted patents WO 2013/091001; WO 2017/035587; WO 2017/015703; WO 2021/119727. All other authors declare no competing interest.
Figures



References
-
- Sun Y., Nan D., Jin H., Qu X., Polym. Test. 2020, 81, 106283.
-
- Rizzo F., Kehr N. S., Adv. Healthcare Mater. 2021, 10, 2001341. - PubMed
-
- Busscher H. J., van der Mei H. C., Subbiahdoss G., Jutte P. C., van den Dungen J. J. A. M., Zaat S. A. J., Schultz M. J., Grainger D. W., Sci. Transl. Med. 2012, 4. - PubMed
-
- Carragee E. J., Hurwitz E. L., Weiner B. K., Spine J. 2011, 11, 471. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

