Domain generalization for prostate segmentation in transrectal ultrasound images: A multi-center study
- PMID: 36148705
- PMCID: PMC10161676
- DOI: 10.1016/j.media.2022.102620
Domain generalization for prostate segmentation in transrectal ultrasound images: A multi-center study
Abstract
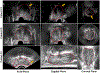
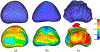
Prostate biopsy and image-guided treatment procedures are often performed under the guidance of ultrasound fused with magnetic resonance images (MRI). Accurate image fusion relies on accurate segmentation of the prostate on ultrasound images. Yet, the reduced signal-to-noise ratio and artifacts (e.g., speckle and shadowing) in ultrasound images limit the performance of automated prostate segmentation techniques and generalizing these methods to new image domains is inherently difficult. In this study, we address these challenges by introducing a novel 2.5D deep neural network for prostate segmentation on ultrasound images. Our approach addresses the limitations of transfer learning and finetuning methods (i.e., drop in performance on the original training data when the model weights are updated) by combining a supervised domain adaptation technique and a knowledge distillation loss. The knowledge distillation loss allows the preservation of previously learned knowledge and reduces the performance drop after model finetuning on new datasets. Furthermore, our approach relies on an attention module that considers model feature positioning information to improve the segmentation accuracy. We trained our model on 764 subjects from one institution and finetuned our model using only ten subjects from subsequent institutions. We analyzed the performance of our method on three large datasets encompassing 2067 subjects from three different institutions. Our method achieved an average Dice Similarity Coefficient (Dice) of 94.0±0.03 and Hausdorff Distance (HD95) of 2.28 mm in an independent set of subjects from the first institution. Moreover, our model generalized well in the studies from the other two institutions (Dice: 91.0±0.03; HD95: 3.7 mm and Dice: 82.0±0.03; HD95: 7.1 mm). We introduced an approach that successfully segmented the prostate on ultrasound images in a multi-center study, suggesting its clinical potential to facilitate the accurate fusion of ultrasound and MRI images to drive biopsy and image-guided treatments.
Keywords: Continual learning segmentation; Deep learning; Gland segmentation; Prostate MRI; Targeted biopsy; Transrectal ultrasound.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
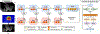
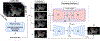




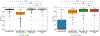




References
-
- Anas EMA, Mousavi P, Abolmaesumi P, 2018. A deep learning approach for real time prostate segmentation in freehand ultrasound guided biopsy. Medical Image Analysis 48, 107–116. - PubMed
-
- Anas EMA, Nouranian S, Mahdavi SS, Spadinger I, Morris WJ, Salcudean SE, Mousavi P, Abolmaesumi P, 2017. clinical target-volume delineation in prostate brachytherapy using residual neural networks, in: Medical Image Computing and Computer Assisted Intervention, MICCAI 2017, pp. 365–373.
-
- Avola D, Cinque L, Fagioli A, Foresti G, Mecca A, 2021. Ultrasound medical imaging techniques: A survey. ACM Comput. Surv 54.
-
- Azizi S, Van Woudenberg N, Sojoudi S, Li M, Xu S, Abu Anas EM, Yan P, Tahmasebi A, Kwak JT, Turkbey B, Choyke P, Pinto P, Wood B, Mousavi P, Abolmaesumi P, 2018. Toward a real-time system for temporal enhanced ultrasound-guided prostate biopsy. International Journal of Computer Assisted Radiology and Surgery 13, 1201–1209. - PMC - PubMed

