Molecular mechanisms of metabolic associated fatty liver disease (MAFLD): functional analysis of lipid metabolism pathways
- PMID: 36148775
- PMCID: PMC9508552
- DOI: 10.1042/CS20220572
Molecular mechanisms of metabolic associated fatty liver disease (MAFLD): functional analysis of lipid metabolism pathways
Abstract
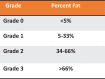
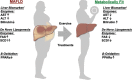
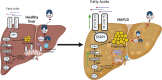
The metabolic-associated fatty liver disease (MAFLD) is a condition of fat accumulation in the liver in combination with metabolic dysfunction in the form of overweight or obesity and insulin resistance. It is also associated with an increased cardiovascular disease risk, including hypertension and atherosclerosis. Hepatic lipid metabolism is regulated by a combination of the uptake and export of fatty acids, de novo lipogenesis, and fat utilization by β-oxidation. When the balance between these pathways is altered, hepatic lipid accumulation commences, and long-term activation of inflammatory and fibrotic pathways can progress to worsen the liver disease. This review discusses the details of the molecular mechanisms regulating hepatic lipids and the emerging therapies targeting these pathways as potential future treatments for MAFLD.
Keywords: hepatic steatosis; intracellular signaling; non alcoholic fatty liver disease; obesity.
© 2022 The Author(s).
Conflict of interest statement
T.D.H.J. and D.E.S. have submitted patents on bilirubin and obesity-related disorders.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

