Effect of RNS60 in amyotrophic lateral sclerosis: a phase II multicentre, randomized, double-blind, placebo-controlled trial
- PMID: 36148821
- PMCID: PMC10092300
- DOI: 10.1111/ene.15573
Effect of RNS60 in amyotrophic lateral sclerosis: a phase II multicentre, randomized, double-blind, placebo-controlled trial
Abstract
Background and purpose: Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease with limited treatment options. RNS60 is an immunomodulatory and neuroprotective investigational product that has shown efficacy in animal models of ALS and other neurodegenerative diseases. Its administration has been safe and well tolerated in ALS subjects in previous early phase trials.
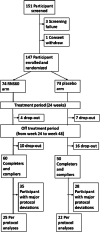
Methods: This was a phase II, multicentre, randomized, double-blind, placebo-controlled, parallel-group trial. Participants diagnosed with definite, probable or probable laboratory-supported ALS were assigned to receive RNS60 or placebo administered for 24 weeks intravenously (375 ml) once a week and via nebulization (4 ml/day) on non-infusion days, followed by an additional 24 weeks off-treatment. The primary objective was to measure the effects of RNS60 treatment on selected biomarkers of inflammation and neurodegeneration in peripheral blood. Secondary objectives were to measure the effect of RNS60 on functional impairment (ALS Functional Rating Scale-Revised), a measure of self-sufficiency, respiratory function (forced vital capacity, FVC), quality of life (ALS Assessment Questionnaire-40, ALSAQ-40) and survival. Tolerability and safety were assessed.
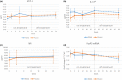
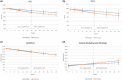
Results: Seventy-four participants were assigned to RNS60 and 73 to placebo. Assessed biomarkers did not differ between arms. The mean rate of decline in FVC and the eating and drinking domain of ALSAQ-40 was slower in the RNS60 arm (FVC, difference 0.41 per week, standard error 0.16, p = 0.0101; ALSAQ-40, difference -0.19 per week, standard error 0.10, p = 0.0319). Adverse events were similar in the two arms. In a post hoc analysis, neurofilament light chain increased over time in bulbar onset placebo participants whilst remaining stable in those treated with RNS60.
Conclusions: The positive effects of RNS60 on selected measures of respiratory and bulbar function warrant further investigation.
Keywords: ALS; clinical trial; placebo-controlled; randomized; treatment.
© 2022 The Authors. European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
E. Beghi reports grants from the Italian Ministry of Health, grants from SOBI, personal fees from Arvelle Therapeutics, outside the submitted work. E. Beghi reports grants from American ALS Association for the conduct of the present study. E. Pupillo reports grants from the Italian Ministry of Health, grants from AIFA, outside the submitted work. A. Sherman reports research grants from the ALS Association, ALS Finding a Cure and the NIH, outside the submitted work. S. Paganoni reports research grants from Amylyx Therapeutics, Revalesio Corporation, UCB/Ra Pharma, Biohaven, Clene, Prilenia, Seelos, the ALS Association, the American Academy of Neurology, ALS Finding a Cure, the Salah Foundation, the Spastic Paraplegia Foundation, the Muscular Dystrophy Association and reports personal consulting fees for advisory panels from Orion and Cytokinetics. All of them are outside the submitted work. M. Filosto reports serving on a scientific advisory board for Amicus, Sanofi, Sarepta; honoraria for speaking engagements for Sanofi, Alnylam. All of them are outside the submitted work.
A. Padovani serves on a scientific advisory board: Director of School of Neurology, University of Brescia; President‐elect of the National Society of Neurology SIN; GE Healthcare, Lilly and Actelion Ltd Pharmaceuticals. Travel funded by a commercial entity: Roche, Ely‐Lilly, Biogen, Zambon, Nutricia, Lundbeck. Serving as a journal editor, associate editor, or on an editorial advisory board: MDPI. Patents held or pending: Method of generation of a diagnostic index for Alzheimer's disease, electronic device for implementing the method and system, 2016. Honoraria for speaking engagements: He has received honoraria from Nutricia, PIAM, Langstone Technology, GE Healthcare, Lilly and Chiesi. Commercial research support: Advisory and served on the Scientific Advisory Board for GE Healthcare, Lilly and Actelion Ltd Pharmaceuticals. Government research support (including funding organization, grant number, and role): He has participated as PI in more than 30 research projects funded by the Ministry of Health and MIUR. Support from a non‐profit foundation or society: CHDI Foundation, Fondazione Cariplo. All of them are outside the submitted work.
G. Logroscino received funds from Roche, Amplifon; he received travel funds for participation in a congress as speaker. All of them are outside the submitted work. C. Lunetta received compensation for consulting services from Neuraltus, Cytokinetics, Mitsubishi Tanabe Pharma Europe and Italfarmaco and has received funds from ARISLA and Italian Ministry of Health. All of them are outside the submitted work. J. Mandrioli received research grants from Fondazione Italiana di Ricerca per la Sclerosi Laterale Amiotrofica (ARISLA), the Agenzia Italiana del Farmaco (AIFA), the Italian Ministry of Health, Emilia Romagna Regional Health Authority, Roche, Pfizer. All of them are outside the submitted work. F. Trojsi served as a journal editor, associate editor, or on an editorial advisory board: Associate Editor of
All other authors report no disclosures.
Figures

References
-
- Beghi E, Mennini T, Bendotti C, et al. The heterogeneity of amyotrophic lateral sclerosis: a possible explanation of treatment failure. Curr Med Chem. 2007;14(30):3185‐3200. - PubMed
-
- Writing Group; Edaravone (MCI‐186) ALS 19 Study Group . Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double‐blind, placebo‐controlled trial. Lancet Neurol. 2017;16(7):505‐512. - PubMed
-
- Calió ML, Henriques E, Siena A, Bertoncini CRA, Gil‐Mohapel J, Rosenstock TR. Mitochondrial dysfunction, neurogenesis, and epigenetics: putative implications for amyotrophic lateral sclerosis neurodegeneration and treatment. Front Neurosci. 2020;14:679. doi:10.3389/fnins.2020.00679 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

