Electroencephalography functional connectivity-A biomarker for painful polyneuropathy
- PMID: 36148823
- PMCID: PMC10092565
- DOI: 10.1111/ene.15575
Electroencephalography functional connectivity-A biomarker for painful polyneuropathy
Abstract
Background and purpose: Advanced analysis of electroencephalography (EEG) data has become an essential tool in brain research. Based solely on resting state EEG signals, a data-driven, predictive and explanatory approach is presented to discriminate painful from non-painful diabetic polyneuropathy (DPN) patients.
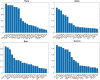
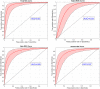
Methods: Three minutes long, 64 electrode resting-state recordings were obtained from 180 DPN patients. The analysis consisted of a mixture of traditional, explanatory and machine learning analyses. First, the 10 functional bivariate connections best differentiating between painful and non-painful patients in each EEG band were identified and the relevant receiver operating characteristic was calculated. Later, those connections were correlated with selected clinical parameters.
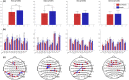
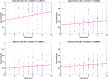
Results: Predictive analysis indicated that theta and beta bands contain most of the information required for discrimination between painful and non-painful polyneuropathy patients, with area under the receiver operating characteristic curve values of 0.93 for theta and 0.89 for beta bands. Assessing statistical differences between the average magnitude of functional connectivity values and clinical pain parameters revealed that painful DPN patients had significantly higher cortical functional connectivity than non-painful ones (p = 0.008 for theta and p = 0.001 for alpha bands). Moreover, intra-band analysis of individual significant functional connections revealed a positive correlation with average reported pain in the previous 3 months in all frequency bands.
Conclusions: Resting state EEG functional connectivity can serve as a highly accurate biomarker for the presence or absence of pain in DPN patients. This highlights the importance of the brain, in addition to the peripheral lesions, in generating the clinical pain picture. This tool can probably be extended to other pain syndromes.
Trial registration: ClinicalTrials.gov NCT02402361.
Keywords: biomarkers for pain; functional connectivity; multivariate analysis; painful polyneuropathy; resting state EEG.
© 2022 The Authors. European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
No authors declare a conflict of interest related to this work.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

