Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD)
- PMID: 36148880
- PMCID: PMC10008140
- DOI: 10.2337/dci22-0034
Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD)
Abstract
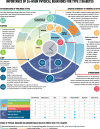
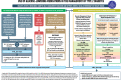
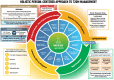
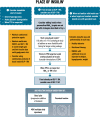
The American Diabetes Association and the European Association for the Study of Diabetes convened a panel to update the previous consensus statements on the management of hyperglycemia in type 2 diabetes in adults, published since 2006 and last updated in 2019. The target audience is the full spectrum of the professional health care team providing diabetes care in the U.S. and Europe. A systematic examination of publications since 2018 informed new recommendations. These include additional focus on social determinants of health, the health care system, and physical activity behaviors, including sleep. There is a greater emphasis on weight management as part of the holistic approach to diabetes management. The results of cardiovascular and kidney outcomes trials involving sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide 1 receptor agonists, including assessment of subgroups, inform broader recommendations for cardiorenal protection in people with diabetes at high risk of cardiorenal disease. After a summary listing of consensus recommendations, practical tips for implementation are provided.
© 2022 by the American Diabetes Association and the European Association for the Study of Diabetes.
Conflict of interest statement
Figures


References
-
- Rodriguez-Gutierrez R, Gionfriddo MR, Ospina NS, et al. Shared decision making in endocrinology: present and future directions. Lancet Diabetes Endocrinol 2016;4:706–716 - PubMed
-
- Draznin B, Aroda VR, Bakris G, et al.; American Diabetes Association Professional Practice Committee . 6. Glycemic targets: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45(Suppl. 1):S83–S96 - PubMed
-
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centred approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577–1596 - PubMed
-
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015;58:429–442 - PubMed
-
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018;61:2461–2498 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

