Shock and kill within the CNS: A promising HIV eradication approach?
- PMID: 36148896
- PMCID: PMC9826147
- DOI: 10.1002/JLB.5VMR0122-046RRR
Shock and kill within the CNS: A promising HIV eradication approach?
Abstract
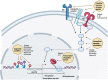
The most studied HIV eradication approach is the "shock and kill" strategy, which aims to reactivate the latent reservoir by latency reversing agents (LRAs) and allowing elimination of these cells by immune-mediated clearance or viral cytopathic effects. The CNS is an anatomic compartment in which (persistent) HIV plays an important role in HIV-associated neurocognitive disorder. Restriction of the CNS by the blood-brain barrier is important for maintenance of homeostasis of the CNS microenvironment, which includes CNS-specific cell types, expression of transcription factors, and altered immune surveillance. Within the CNS predominantly myeloid cells such as microglia and perivascular macrophages are thought to be a reservoir of persistent HIV infection. Nevertheless, infection of T cells and astrocytes might also impact HIV infection in the CNS. Genetic adaptation to this microenvironment results in genetically distinct, compartmentalized viral populations with differences in transcription profiles. Because of these differences in transcription profiles, LRAs might have different effects within the CNS as compared with the periphery. Moreover, reactivation of HIV in the brain and elimination of cells within the CNS might be complex and could have detrimental consequences. Finally, independent of activity on latent HIV, LRAs themselves can have adverse neurologic effects. We provide an extensive overview of the current knowledge on compartmentalized (persistent) HIV infection in the CNS and on the "shock and kill" strategy. Subsequently, we reflect on the impact and promise of the "shock and kill" strategy on the elimination of persistent HIV in the CNS.
Keywords: CNS; HAND; HIV; astrocytes; compartmentalization; eradication; inflammation; latency; latency reversal; microglia; perivascular macrophages; persistence; reservoir; shock and kill.
© 2022 The Authors. Journal of Leukocyte Biology published by Wiley Periodicals LLC on behalf of Society for Leukocyte Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Finzi D, Blankson J, Siliciano JD, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV‐1, even in patients on effective combination therapy. Nature Medicine. 1999;5: 512‐7. - PubMed
-
- Balcom EF, Roda WC, Cohen EA, Li MY, Power C. HIV‐1 persistence in the central nervous system: viral and host determinants during antiretroviral therapy. Current Opinion in Virology. 2019;38: 54‐62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

