Evaluating real-world COVID-19 vaccine effectiveness using a test-negative case-control design
- PMID: 36148919
- PMCID: PMC9504802
- DOI: 10.2217/cer-2022-0069
Evaluating real-world COVID-19 vaccine effectiveness using a test-negative case-control design
Abstract
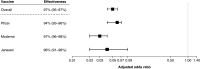
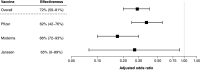
Aim: It is important to assess if clinical trial efficacy translates into real-world effectiveness for COVID-19 vaccines. Materials & methods: We conducted a modified test-negative design (TND) to evaluate the real-world effectiveness of three COVID-19 vaccines. We defined cases in two ways: self-reported COVID-19-positive tests, and self-reported positive tests with ≥1 moderate/severe COVID-19 symptom. Results: Any vaccination was associated with a 95% reduction in subsequently reporting a positive COVID-19 test, and a 71% reduction in reporting a positive test and ≥1 moderate/severe symptom. Conclusion: We observed high effectiveness across all three marketed vaccines, both for self-reported positive COVID-19 tests and moderate/severe COVID-19 symptoms. This innovative TND approach can be implemented in future COVID-19 vaccine and treatment real-world effectiveness studies. Clinicaltrials.gov identifier: NCT04368065.
Keywords: COVID-19; SARS-CoV-2; patient-reported outcomes; person-generated health data; symptoms; test-negative design; vaccines.
Figures
References
-
- Patel MM, Jackson ML, Ferdinands J. Postlicensure evaluation of COVID-19 vaccines. JAMA 324(19), 1939–1940 (2020). - PubMed
-
• Peer-reviewed publication of post-licensure evaluation of COVID-19 vaccines using test-negative design. Findings show that “the test-negative design is popular because it offers 2 advantages: simplified logistics, because controls are already identified when identifying cases, and reduced confounding due to similar health care use patterns between cases and controls, similar to a nested case–control study”.
-
- Chua H, Feng S, Lewnard JA et al. The use of test-negative controls to monitor vaccine effectiveness: a systematic review of methodology. Epidemiology 31(1), 43–64 (2020). - PMC - PubMed
-
• Peer-reviewed publication of methodology of using test-negative design. Findings show that “The test-negative design has been increasingly used as an efficient study design to estimate vaccine effectiveness for a range of vaccines and pathogens”.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
