Viscoelastic Properties of Bioprinted Alginate Microbeads Compared to Their Bulk Hydrogel Analogs
- PMID: 36149022
- PMCID: PMC9791675
- DOI: 10.1115/1.4055757
Viscoelastic Properties of Bioprinted Alginate Microbeads Compared to Their Bulk Hydrogel Analogs
Abstract
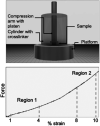
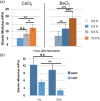
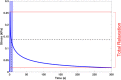
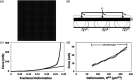
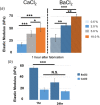
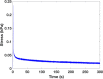
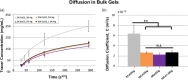
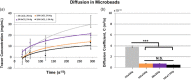
Hydrogel microbeads are engineered spherical microgels widely used for biomedical applications in cell cultures, tissue engineering, and drug delivery. Their mechanical and physical properties (i.e., modulus, porosity, diffusion) heavily influence their utility by affecting encapsulated cellular behavior, biopayload elution kinetics, and stability for longer term cultures. There is a need to quantify these properties to guide microbead design for effective application. However, there are few techniques with the μN-level resolution required to evaluate these relatively small, compliant constructs. To circumvent mechanically testing individual microbeads, researchers often approximate microbead properties by characterizing larger bulk gel analogs of the same material formulation. This approach provides some insight into the hydrogel properties. However, bulk gels possess key structural and mechanical differences compared to their microbead equivalents, which may limit their accuracy and utility as analogs for estimating microbead properties. Herein, we explore how microbead properties are influenced by hydrogel formulation (i.e., alginate concentration, divalent cation crosslinker, and crosslinker concentration), and whether these trends are accurately reflected in bulk gel analogs. To accomplish this, we utilize laser direct-write bioprinting to create 12 × 12 arrays of alginate microbeads and characterize all 144 microbeads in parallel using a commercially available microcompression system. In this way, the compressive load is distributed across a large number of beads, thus amplifying sample signal. Comparing microbead properties to those of their bulk gel analogs, we found that their trends in modulus, porosity, and diffusion with hydrogel formulation are consistent, yet bulk gels exhibit significant discrepancies in their measured values.
Keywords: bulk gel; hydrogel; laser direct-write bioprinting; microbead; microcompression.
Copyright © 2023 by ASME.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

