Cost Effectiveness of Pharmacogenetic Testing for Drugs with Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines: A Systematic Review
- PMID: 36149409
- PMCID: PMC9828439
- DOI: 10.1002/cpt.2754
Cost Effectiveness of Pharmacogenetic Testing for Drugs with Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines: A Systematic Review
Abstract
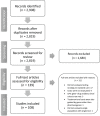
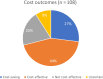
The objective of this study was to evaluate the evidence on cost-effectiveness of pharmacogenetic (PGx)-guided treatment for drugs with Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines. A systematic review was conducted using multiple biomedical literature databases from inception to June 2021. Full articles comparing PGx-guided with nonguided treatment were included for data extraction. Quality of Health Economic Studies (QHES) was used to assess robustness of each study (0-100). Data are reported using descriptive statistics. Of 108 studies evaluating 39 drugs, 77 (71%) showed PGx testing was cost-effective (CE) (N = 48) or cost-saving (CS) (N = 29); 21 (20%) were not CE; 10 (9%) were uncertain. Clopidogrel had the most articles (N = 23), of which 22 demonstrated CE or CS, followed by warfarin (N = 16), of which 7 demonstrated CE or CS. Of 26 studies evaluating human leukocyte antigen (HLA) testing for abacavir (N = 8), allopurinol (N = 10), or carbamazepine/phenytoin (N = 8), 15 demonstrated CE or CS. Nine of 11 antidepressant articles demonstrated CE or CS. The median QHES score reflected high-quality studies (91; range 48-100). Most studies evaluating cost-effectiveness favored PGx testing. Limited data exist on cost-effectiveness of preemptive and multigene testing across disease states.
© 2022 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures
References
-
- US Food and Drug Administration Table of pharmacogenomic biomarkers in drug labeling (U.S. Food and Drug Administration; ). https://www.fda.gov/drugs/science‐and‐research‐drugs/table‐pharmacogenom... Accessed June 24, 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

