Persistence with tamoxifen and aromatase inhibitors in Germany: a retrospective cohort study with 284,383 patients
- PMID: 36149512
- PMCID: PMC10349696
- DOI: 10.1007/s00432-022-04376-5
Persistence with tamoxifen and aromatase inhibitors in Germany: a retrospective cohort study with 284,383 patients
Abstract
Purpose: The aim of this study was to analyze the persistence of women on tamoxifen (TAM) and aromatase inhibitors (AIs) in Germany, and to investigate possible determinants of non-persistence.
Methods: The present retrospective cohort study was based on the IQVIA longitudinal prescription database (LRx). The study included women with an initial prescription of TAM or AIs (anastrozole, letrozole, and exemestane) between January 2016 and December 2020 (index date). Kaplan-Meier analyses were performed to show the persistence for TAM and AI, using a therapy gap of 90 or 180 days, respectively. A multivariable Cox proportional hazards regression model was further used to estimate the relationship between non-persistence and drug prescription (AI versus TAM), age, and the specialty of the physician initiating therapy (gynecologist, oncologist, or general practitioner).
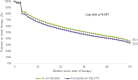
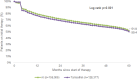
Results: Up to 5 years after the index date, only 35.1% of AI and 32.5% of TAM patients were continuing therapy when therapy discontinuation was defined as at least 90 days without therapy. Using a 180-day therapy gap, 51.9% of AI and 50.4% of TAM patients remained on therapy after 5 years. Cox regression models reveal that initial therapy with TAM (HR 1.06, 95% CI 1.04-1.07), therapy initiation by oncologists (HR 1.09, 95% CI 1.07-1.11), or general practitioners (HR 1.24, 95% CI 1.21-1.27) and age ≤ 50 (HR 1.08, 95% CI 1.06-1.10) were significantly associated with an increased risk of therapy discontinuation.
Conclusion: Overall, the present study indicates that persistence rates are low in all age groups for both TAM and AI treatment. We found several factors (e.g., physician specialty, younger age, and type of endocrine therapy) to be associated with an increased risk for non-persistence.
Keywords: Aromatase inhibitors; Breast cancer; Endocrine therapy; Germany; Persistence; Tamoxifen.
© 2022. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures
Similar articles
-
Hormonal therapies for early breast cancer: systematic review and economic evaluation.Health Technol Assess. 2007 Jul;11(26):iii-iv, ix-xi, 1-134. doi: 10.3310/hta11260. Health Technol Assess. 2007. PMID: 17610808
-
Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD003370. doi: 10.1002/14651858.CD003370.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2009 Oct 07;(4):CD003370. doi: 10.1002/14651858.CD003370.pub3. PMID: 17253488 Updated.
-
Systematic review of aromatase inhibitors in the first-line treatment for hormone sensitive advanced or metastatic breast cancer.Breast Cancer Res Treat. 2010 Aug;123(1):9-24. doi: 10.1007/s10549-010-0974-0. Epub 2010 Jun 10. Breast Cancer Res Treat. 2010. PMID: 20535542
-
Risk-reducing medications for primary breast cancer: a network meta-analysis.Cochrane Database Syst Rev. 2019 Apr 29;4(4):CD012191. doi: 10.1002/14651858.CD012191.pub2. Cochrane Database Syst Rev. 2019. PMID: 31032883 Free PMC article.
-
Mammographic density, endocrine therapy and breast cancer risk: a prognostic and predictive biomarker review.Cochrane Database Syst Rev. 2021 Oct 26;10(10):CD013091. doi: 10.1002/14651858.CD013091.pub2. Cochrane Database Syst Rev. 2021. PMID: 34697802 Free PMC article.
Cited by
-
Systematic literature review and trial-level meta-analysis of aromatase inhibitors vs tamoxifen in patients with HR+/HER2- early breast cancer.Breast. 2025 Jun;81:104429. doi: 10.1016/j.breast.2025.104429. Epub 2025 Mar 5. Breast. 2025. PMID: 40153939 Free PMC article.
-
Extended endocrine therapy use and decision making after breast cancer diagnosis.J Natl Cancer Inst. 2025 Aug 1;117(8):1573-1582. doi: 10.1093/jnci/djaf076. J Natl Cancer Inst. 2025. PMID: 40163701 Free PMC article.
-
Initiation of Antiresorptive Drug Treatment during Endocrine Therapy for Breast Cancer-A Retrospective Cohort Study of 161,492 Patients in Germany.Cancers (Basel). 2023 Mar 19;15(6):1847. doi: 10.3390/cancers15061847. Cancers (Basel). 2023. PMID: 36980733 Free PMC article.
-
Association between gout and subsequent breast cancer: a retrospective cohort study including 67,598 primary care patients in Germany.Breast Cancer Res Treat. 2023 Jun;199(3):545-552. doi: 10.1007/s10549-023-06944-w. Epub 2023 Apr 18. Breast Cancer Res Treat. 2023. PMID: 37071268 Free PMC article.
-
Antibiotic Prescription in Dentistry: Trends, Patient Demographics, and Drug Preferences in Germany.Antibiotics (Basel). 2025 Jul 3;14(7):676. doi: 10.3390/antibiotics14070676. Antibiotics (Basel). 2025. PMID: 40723979 Free PMC article.
References
-
- Barnes B, Kraywinkel K, Nowossadeck E, Schönfeld I, Starker A, Wienecke A, Wolf U (2016) Bericht zum Krebsgeschehen in Deutschland 2016. Robert Koch-Institut, Berlin
-
- Blanchette PS, Lam M, Richard L, Allen B, Shariff SZ, Vandenberg T, Pritchard KI, Chan KKW, Louie AV, Desautels D, Raphael J, Earle CC (2020) Factors associated with endocrine therapy adherence among post-menopausal women treated for early-stage breast cancer in Ontario, Canada. Breast Cancer Res Treat 179(1):217–227. 10.1007/s10549-019-05430-6 - PubMed
-
- Collin LJ, Cronin-Fenton DP, Ahern TP, Goodman M, McCullough LE, Waller LA, Kjaersgaard A, Damkier P, Christiansen PM, Ejlertsen B, Jensen MB, Sorensen HT, Lash TL (2021) Early discontinuation of endocrine therapy and recurrence of breast cancer among premenopausal women. Clin Cancer Res 27(5):1421–1428. 10.1158/1078-0432.CCR-20-3974 - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

