Non-pharmacological interventions for vascular health and the role of the endothelium
- PMID: 36149520
- PMCID: PMC9613570
- DOI: 10.1007/s00421-022-05041-y
Non-pharmacological interventions for vascular health and the role of the endothelium
Abstract
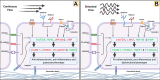
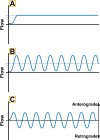
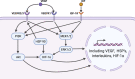
The most common non-pharmacological intervention for both peripheral and cerebral vascular health is regular physical activity (e.g., exercise training), which improves function across a range of exercise intensities and modalities. Numerous non-exercising approaches have also been suggested to improved vascular function, including repeated ischemic preconditioning (IPC); heat therapy such as hot water bathing and sauna; and pneumatic compression. Chronic adaptive responses have been observed across a number of these approaches, yet the precise mechanisms that underlie these effects in humans are not fully understood. Acute increases in blood flow and circulating signalling factors that induce responses in endothelial function are likely to be key moderators driving these adaptations. While the impact on circulating factors and environmental mechanisms for adaptation may vary between approaches, in essence, they all centre around acutely elevating blood flow throughout the circulation and stimulating improved endothelium-dependent vascular function and ultimately vascular health. Here, we review our current understanding of the mechanisms driving endothelial adaptation to repeated exposure to elevated blood flow, and the interplay between this response and changes in circulating factors. In addition, we will consider the limitations in our current knowledge base and how these may be best addressed through the selection of more physiologically relevant experimental models and research. Ultimately, improving our understanding of the unique impact that non-pharmacological interventions have on the vasculature will allow us to develop superior strategies to tackle declining vascular function across the lifespan, prevent avoidable vascular-related disease, and alleviate dependency on drug-based interventions.
Keywords: Endothelial; Exercise; Heat; Hypoxia; Ischemic preconditioning; Non-pharmacological intervention; Vascular function.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. - PubMed
-
- Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100:174–190. - PubMed
-
- Akerman AP, Thomas KN, van Rij AM, Body ED, Alfadhel M, Cotter JD. Heat therapy vs. supervised exercise therapy for peripheral arterial disease: a 12-wk randomized, controlled trial. Am J Physiol Hear Circ Physiol. 2019;316:H1495–H1506. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

