Feasibility, Acceptability, and Protective Efficacy of Seasonal Malaria Chemoprevention Implementation in Nampula Province, Mozambique: Protocol for a Hybrid Effectiveness-Implementation Study
- PMID: 36149743
- PMCID: PMC9547334
- DOI: 10.2196/36403
Feasibility, Acceptability, and Protective Efficacy of Seasonal Malaria Chemoprevention Implementation in Nampula Province, Mozambique: Protocol for a Hybrid Effectiveness-Implementation Study
Abstract
Background: Seasonal malaria chemoprevention (SMC) is a highly effective community-based intervention to prevent malaria infections in areas where the malaria burden is high and transmission occurs mainly during the rainy season. In Africa, so far, SMC has been implemented in the Sahel region. Mozambique contributes 4% of the global malaria cases, and malaria is responsible for one-quarter of all deaths in the country. Based on recommendations in the Malaria Strategic Plan, the Malaria Consortium, in partnership with the National Malaria Control Programme in Mozambique, initiated a phased SMC implementation study in the northern province of Nampula. The first phase of this 2-year implementation study was conducted in 2020-2021 and focused on the feasibility and acceptability of SMC. The second phase will focus on demonstrating impact. This paper describes phase 2 of the implementation study.
Objective: Specific objectives include the following: (1) to determine the effectiveness of SMC in terms of its reduction in incidence of malaria infection among children aged 3 to 59 months; (2) to determine the chemoprevention efficacy of sulfadoxine-pyrimethamine plus amodiaquine (SP+AQ) when used for SMC in Nampula Province, Mozambique, and the extent to which efficacy is impacted by drug resistance and drug concentrations; (3) to investigate the presence and change in SP+AQ- and piperaquine-resistance markers over time as a result of SMC implementation; and (4) to understand the impact of the SMC implementation model, determining the process and acceptability outcomes for the intervention.
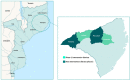
Methods: This type 2, hybrid, effectiveness-implementation study uses a convergent mixed methods approach. SMC will be implemented in four monthly cycles between December 2021 and March 2022 in four districts of Nampula Province. Phase 2 will include four components: (1) a cluster randomized controlled trial to establish confirmed malaria cases, (2) a prospective cohort to determine the chemoprevention efficacy of the antimalarials used for SMC and whether drug concentrations or resistance influence the duration of protection, (3) a resistance marker study in children aged 3 to 59 months to describe changes in resistance marker prevalence over time, and (4) a process evaluation to determine feasibility and acceptability of SMC.
Results: Data collection began in mid-January 2022, and data analysis is expected to be completed by October 2022.
Conclusions: This is the first effectiveness trial of SMC implemented in Mozambique. The findings from this trial will be crucial to policy change and program expansion to other suitable geographies outside of the Sahel. The chemoprevention efficacy cohort study is a unique opportunity to better understand SMC drug efficacy in this new SMC environment.
Trial registration: ClinicalTrials.gov NCT05186363; https://clinicaltrials.gov/ct2/show/NCT05186363.
International registered report identifier (irrid): DERR1-10.2196/36403.
Keywords: Mozambique; Nampula; SMC; SP+AQ; cRCT; chemoprevention; children; effectiveness; feasibility; hybrid effectiveness; malaria; mixed methods; protocol.
©Kevin Baker, Pedro Aide, Craig A Bonnington, Christian Rassi, Sol Richardson, Arantxa Roca-Feltrer, Maria Rodrigues, Mercia Sitoe, Ivan Alejandro Pulido Tarquino, Sonia Enosse, Caitlin McGugan, Eva Amelia de Carvalho, Francisco Saute, Alfredo Gabriel Mayor Aparicio, Baltazar Candrinho. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 23.09.2022.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

Similar articles
-
A hybrid effectiveness-implementation study protocol to assess the effectiveness and chemoprevention efficacy of implementing seasonal malaria chemoprevention in five districts in Karamoja region, Uganda.Gates Open Res. 2023 Dec 18;7:14. doi: 10.12688/gatesopenres.14287.2. eCollection 2023. Gates Open Res. 2023. PMID: 38196920 Free PMC article.
-
Phase one of a hybrid effectiveness-implementation study to assess the feasibility, acceptability and effectiveness of implementing seasonal malaria chemoprevention in Nampula Province, Mozambique.Malar J. 2025 Feb 21;24(1):56. doi: 10.1186/s12936-024-05229-x. Malar J. 2025. PMID: 39985013 Free PMC article.
-
Assessment of the Feasibility, Acceptability, and Impact of Implementing Seasonal Malaria Chemoprevention in Nampula Province, Mozambique: Protocol for a Hybrid Effectiveness-Implementation Study.JMIR Res Protoc. 2021 Sep 15;10(9):e27855. doi: 10.2196/27855. JMIR Res Protoc. 2021. PMID: 34524109 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Effect of seasonal malaria chemoprevention in children between 5 and 9 years old in Kita and Bafoulabe districts, Mali.Parasite Epidemiol Control. 2022 Jun 22;18:e00258. doi: 10.1016/j.parepi.2022.e00258. eCollection 2022 Aug. Parasite Epidemiol Control. 2022. PMID: 35789762 Free PMC article. Review.
Cited by
-
A hybrid effectiveness-implementation study protocol to assess the effectiveness and chemoprevention efficacy of implementing seasonal malaria chemoprevention in five districts in Karamoja region, Uganda.Gates Open Res. 2023 Dec 18;7:14. doi: 10.12688/gatesopenres.14287.2. eCollection 2023. Gates Open Res. 2023. PMID: 38196920 Free PMC article.
-
Phase one of a hybrid effectiveness-implementation study to assess the feasibility, acceptability and effectiveness of implementing seasonal malaria chemoprevention in Nampula Province, Mozambique.Malar J. 2025 Feb 21;24(1):56. doi: 10.1186/s12936-024-05229-x. Malar J. 2025. PMID: 39985013 Free PMC article.
-
Field testing of user-friendly perennial malaria chemoprevention packaging in Benin, Côte d'Ivoire and Mozambique.Malar J. 2024 May 21;23(1):157. doi: 10.1186/s12936-024-04977-0. Malar J. 2024. PMID: 38773567 Free PMC article.
-
Malaria trends in districts that were targeted and not-targeted for seasonal malaria chemoprevention in children under 5 years of age in Guinea, 2014-2021.BMJ Glob Health. 2024 Feb 26;9(2):e013898. doi: 10.1136/bmjgh-2023-013898. BMJ Glob Health. 2024. PMID: 38413098 Free PMC article.
-
Seasonal malaria chemoprevention in northern Mozambique: a cost-effectiveness analysis.Malar J. 2025 May 21;24(1):159. doi: 10.1186/s12936-025-05401-x. Malar J. 2025. PMID: 40399941 Free PMC article.
References
-
- Seasonal Malaria Chemoprevention With Sulfadoxine-Pyrimethamine Plus Amodiaquine in Children. A Field Guide. Geneva, Switzerland: World Health Organization; 2013. Jul, [2022-07-21]. https://apps.who.int/iris/bitstream/handle/10665/85726/9789241504737_eng... .
-
- ACCESS-SMC Partnership Effectiveness of seasonal malaria chemoprevention at scale in west and central Africa: An observational study. Lancet. 2020 Dec 05;396(10265):1829–1840. doi: 10.1016/S0140-6736(20)32227-3. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(20)32227-3 S0140-6736(20)32227-3 - DOI - PMC - PubMed
-
- Cissé B, Sokhna C, Boulanger D, Milet J, Bâ EH, Richardson K, Hallett R, Sutherland C, Simondon K, Simondon F, Alexander N, Gaye O, Targett G, Lines J, Greenwood B, Trape J. Seasonal intermittent preventive treatment with artesunate and sulfadoxine-pyrimethamine for prevention of malaria in Senegalese children: A randomised, placebo-controlled, double-blind trial. Lancet. 2006 Feb 25;367(9511):659–667. doi: 10.1016/S0140-6736(06)68264-0.S0140-6736(06)68264-0 - DOI - PubMed
-
- Dicko A, Diallo AI, Tembine I, Dicko Y, Dara N, Sidibe Y, Santara G, Diawara H, Conaré T, Djimde A, Chandramohan D, Cousens S, Milligan PJ, Diallo DA, Doumbo OK, Greenwood B. Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated bednet in Mali: A randomised, double-blind, placebo-controlled trial. PLoS Med. 2011 Feb 01;8(2):e1000407. doi: 10.1371/journal.pmed.1000407. https://dx.plos.org/10.1371/journal.pmed.1000407 - DOI - DOI - PMC - PubMed
-
- Gilmartin C, Nonvignon J, Cairns M, Milligan P, Bocoum F, Winskill P, Moroso D, Collins D. Seasonal malaria chemoprevention in the Sahel subregion of Africa: A cost-effectiveness and cost-savings analysis. Lancet Glob Health. 2021 Feb;9(2):e199–e208. doi: 10.1016/S2214-109X(20)30475-7. https://linkinghub.elsevier.com/retrieve/pii/S2214-109X(20)30475-7 S2214-109X(20)30475-7 - DOI - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

