Excited-State Barrier Controls E → Z Photoisomerization in p-Hydroxycinnamate Biochromophores
- PMID: 36149746
- PMCID: PMC9549896
- DOI: 10.1021/acs.jpclett.2c02613
Excited-State Barrier Controls E → Z Photoisomerization in p-Hydroxycinnamate Biochromophores
Abstract
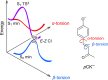
Molecules based on the deprotonated p-hydroxycinnamate moiety are widespread in nature, including serving as UV filters in the leaves of plants and as the biochromophore in photoactive yellow protein. The photophysical behavior of these chromophores is centered around a rapid E → Z photoisomerization by passage through a conical intersection seam. Here, we use photoisomerization and photodissociation action spectroscopies with deprotonated 4-hydroxybenzal acetone (pCK-) to characterize a wavelength-dependent bifurcation between electron autodetachment (spontaneous ejection of an electron from the S1 state because it is situated in the detachment continuum) and E → Z photoisomerization. While autodetachment occurs across the entire S1(ππ*) band (370-480 nm), E → Z photoisomerization occurs only over a blue portion of the band (370-430 nm). No E → Z photoisomerization is observed when the ketone functional group in pCK- is replaced with an ester or carboxylic acid. The wavelength-dependent bifurcation is consistent with potential energy surface calculations showing that a barrier separates the Franck-Condon region from the E → Z isomerizing conical intersection. The barrier height, which is substantially higher in the gas phase than in solution, depends on the functional group and governs whether E → Z photoisomerization occurs more rapidly than autodetachment.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
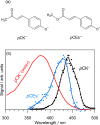
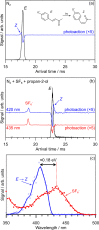

 in a series of alcohols (red) and water–ethylene
glycol mixtures (blue).
in a series of alcohols (red) and water–ethylene
glycol mixtures (blue).References
-
- Holt E. L.; Stavros V. G. Applications of Ultrafast Spectroscopy to Sunscreen Development, From First Principles to Complex Mixtures. Int. Rev. Phys. Chem. 2019, 38, 243–285. 10.1080/0144235X.2019.1663062. - DOI
-
- Kinoshita S.; Harabuchi Y.; Inokuchi Y.; Maeda S.; Ehara M.; Yamazaki K.; Ebata T. Substitution Effect on the Nonradiative Decay and trans → cis Photoisomerization Route: A Guideline to Develop Efficient Cinnamate-Based Sunscreens. Phys. Chem. Chem. Phys. 2021, 23, 834–845. 10.1039/D0CP04402D. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

