Endothelial cell-specific loss of eNOS differentially affects endothelial function
- PMID: 36149900
- PMCID: PMC9506615
- DOI: 10.1371/journal.pone.0274487
Endothelial cell-specific loss of eNOS differentially affects endothelial function
Abstract
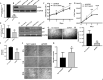
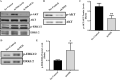
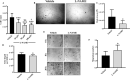
The endothelium maintains and regulates vascular homeostasis mainly by balancing interplay between vasorelaxation and vasoconstriction via regulating Nitric Oxide (NO) availability. Endothelial nitric oxide synthase (eNOS) is one of three NOS isoforms that catalyses the synthesis of NO to regulate endothelial function. However, eNOS's role in the regulation of endothelial function, such as cell proliferation and migration remain unclear. To gain a better understanding, we genetically knocked down eNOS in cultured endothelial cells using sieNOS and evaluated cell proliferation, migration and also tube forming potential in vitro. To our surprise, loss of eNOS significantly induced endothelial cell proliferation, which was associated with significant downregulation of both cell cycle inhibitor p21 and cell proliferation antigen Ki-67. Knockdown of eNOS induced cell migration but inhibited formation of tube-like structures in vitro. Mechanistically, loss of eNOS was associated with activation of MAPK/ERK and inhibition of PI3-K/AKT signaling pathway. On the contrary, pharmacologic inhibition of eNOS by inhibitors L-NAME or L-NMMA, inhibited cell proliferation. Genetic and pharmacologic inhibition of eNOS, both promoted endothelial cell migration but inhibited tube-forming potential. Our findings confirm that eNOS regulate endothelial function by inversely controlling endothelial cell proliferation and migration, and by directly regulating its tube-forming potential. Differential results obtained following pharmacologic versus genetic inhibition of eNOS indicates a more complex mechanism behind eNOS regulation and activity in endothelial cells, warranting further investigation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

