One-Year Mental and Physical Health Assessment in Survivors after Extracorporeal Membrane Oxygenation for COVID-19-related Acute Respiratory Distress Syndrome
- PMID: 36150112
- PMCID: PMC9893333
- DOI: 10.1164/rccm.202206-1145OC
One-Year Mental and Physical Health Assessment in Survivors after Extracorporeal Membrane Oxygenation for COVID-19-related Acute Respiratory Distress Syndrome
Abstract
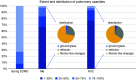
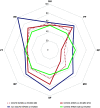
Rationale: Long-term outcomes of patients with coronavirus disease (COVID-19)-related acute respiratory distress syndrome treated with extracorporeal membrane oxygenation (ECMO) are unknown. Objectives: To assess physical examination, pulmonary function tests, anxiety, depression, post-traumatic stress disorder and quality of life at 6 and 12 months after ECMO onset. Methods: Multicenter, prospective study in patients who received ECMO for COVID-19 acute respiratory distress syndrome from March to June 2020 and survived hospital discharge. Measurements and Main Results: Of 80 eligible patients, 62 were enrolled in seven French ICUs. ECMO and invasive mechanical ventilation duration were 18 (11-25) and 36 (27-62) days, respectively. All were alive, but only 19/50 (38%) returned to work and 13/42 (31%) had recovered a normal sex drive at 1 year. Pulmonary function tests were almost normal at 6 months, except for DlCO, which was still impaired at 12 months. Mental health, role-emotional, and role-physical were the most impaired domain compared with patients receiving ECMO who did not have COVID-19. One year after ICU admission, 19/43 (44%) patients had significant anxiety, 18/43 (42%) had depression symptoms, and 21/50 (42%) were at risk for post-traumatic stress disorders. Conclusions: Despite the partial recovery of the lung function tests at 1 year, the physical and psychological function of this population remains impaired. Based on the comparison with long-term follow-up of patients receiving ECMO who did not have COVID-19, poor mental and physical health may be more related to COVID-19 than to ECMO in itself, although this needs confirmation.
Keywords: COVID-19; acute respiratory distress syndrome; follow-up studies; quality of life; venovenous–extracorporeal membrane oxygenation.
Figures

Comment in
-
COVID-19 Extracorporeal Membrane Oxygenation Outcomes: Reporting One-Year Multidimensional Outcomes as Standard Practice.Am J Respir Crit Care Med. 2023 Jan 15;207(2):120-122. doi: 10.1164/rccm.202210-1952ED. Am J Respir Crit Care Med. 2023. PMID: 36301934 Free PMC article. No abstract available.
References
-
- Schmidt M, Hajage D, Lebreton G, Monsel A, Voiriot G, Levy D, et al. Groupe de Recherche Clinique en REanimation et Soins intensifs du Patient en Insuffisance Respiratoire aiguE (GRC-RESPIRE) Sorbonne Université Paris-Sorbonne ECMO-COVID investigators. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med . 2020;8:1121–1131. - PMC - PubMed
-
- Barbaro RP, MacLaren G, Boonstra PS, Combes A, Agerstrand C, Annich G, et al. Extracorporeal Life Support Organization Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet . 2021;398:1230–1238. - PMC - PubMed
-
- Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. CESAR trial collaboration Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet . 2009;374:1351–1363. - PubMed
-
- Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. EOLIA Trial Group, REVA, and ECMONet Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med . 2018;378:1965–1975. - PubMed
-
- Goligher EC, Tomlinson G, Hajage D, Wijeysundera DN, Fan E, Jüni P, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc Bayesian analysis of a randomized clinical trial. JAMA . 2018;320:2251–2259. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

