Molecular detection of SARS-COV-2 in exhaled breath at the point-of-need
- PMID: 36150327
- PMCID: PMC9424122
- DOI: 10.1016/j.bios.2022.114663
Molecular detection of SARS-COV-2 in exhaled breath at the point-of-need
Abstract
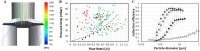
The SARS-CoV-2 pandemic has highlighted the need for improved technologies to help control the spread of contagious pathogens. While rapid point-of-need testing plays a key role in strategies to rapidly identify and isolate infectious patients, current test approaches have significant shortcomings related to assay limitations and sample type. Direct quantification of viral shedding in exhaled particles may offer a better rapid testing approach, since SARS-CoV-2 is believed to spread mainly by aerosols. It assesses contagiousness directly, the sample is easy and comfortable to obtain, sampling can be standardized, and the limited sample volume lends itself to a fast and sensitive analysis. In view of these benefits, we developed and tested an approach where exhaled particles are efficiently sampled using inertial impaction in a micromachined silicon chip, followed by an RT-qPCR molecular assay to detect SARS-CoV-2 shedding. Our portable, silicon impactor allowed for the efficient capture (>85%) of respiratory particles down to 300 nm without the need for additional equipment. We demonstrate using both conventional off-chip and in-situ PCR directly on the silicon chip that sampling subjects' breath in less than a minute yields sufficient viral RNA to detect infections as early as standard sampling methods. A longitudinal study revealed clear differences in the temporal dynamics of viral load for nasopharyngeal swab, saliva, breath, and antigen tests. Overall, after an infection, the breath-based test remains positive during the first week but is the first to consistently report a negative result, putatively signalling the end of contagiousness and further emphasizing the potential of this tool to help manage the spread of airborne respiratory infections.
Keywords: Aerosols; Breath; Diagnostics; Impactor; Lab-on-a-chip; SARS-CoV-2.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Bullard J., Dust K., Funk D., Strong J.E., Alexander D., Garnett L., Boodman C., Bello A., Hedley A., Schiffman Z., Doan K., Bastien N., Li Y., van Caeseele P.G., Poliquin G. Predicting infectious severe acute respiratory syndrome coronavirus 2 from diagnostic samples. Clin. Infect. Dis. 2020;71:2663–2666. doi: 10.1093/cid/ciaa638. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

