Evolution of Care and Outcomes Across Surges in Hospitalized Patients with Coronavirus Disease 2019
- PMID: 36150511
- PMCID: PMC9489963
- DOI: 10.1016/j.amjmed.2022.08.035
Evolution of Care and Outcomes Across Surges in Hospitalized Patients with Coronavirus Disease 2019
Abstract
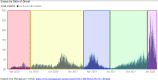
Background: The coronavirus disease 2019 (COVID-19) pandemic has unfolded in distinct surges. Understanding how surges differ may reveal important insights into the evolution of the pandemic and improve patient care.
Methods: We leveraged the Michigan Medicine COVID-19 Cohort, a prospective observational study at an academic tertiary medical center that systematically enrolled 2309 consecutive patients hospitalized for COVID-19, comprising 5 distinct surges.
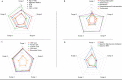
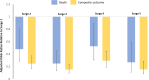
Results: As the pandemic evolved, patients hospitalized for COVID-19 tended to have a lower burden of comorbidities and a lower inflammatory burden as measured by admission levels of C-reactive protein, ferritin, lactate dehydrogenase, and D-dimer. Use of hydroxychloroquine and azithromycin decreased substantially after Surge 1, while use of corticosteroids and remdesivir markedly increased (P < .001 for all). In-hospital mortality significantly decreased from 18.3% in Surge 1 to 5.3% in Surge 5 (P < .001). The need for mechanical ventilation significantly decreased from 42.5% in Surge 1 to 7.0% in Surge 5 (P < .001), while the need for renal replacement therapy decreased from 14.4% in Surge 1 to 2.3% in Surge 5 (P < .001). Differences in patient characteristics, treatments, and inflammatory markers accounted only partially for the differences in outcomes between surges.
Conclusions: The COVID-19 pandemic has evolved significantly with respect to hospitalized patient populations and therapeutic approaches, and clinical outcomes have substantially improved. Hospitalization after the first surge was independently associated with improved outcomes, even after controlling for relevant clinical covariates.
Keywords: Azithromycin; COVID-19; Corticosteroids; Dexamethasone; Hydroxychloroquine; Outcomes; Remdesivir; Surge; Tocilizumab.
Copyright © 2022 Elsevier Inc. All rights reserved.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

