α-Syn overexpression, NRF2 suppression, and enhanced ferroptosis create a vicious cycle of neuronal loss in Parkinson's disease
- PMID: 36150560
- PMCID: PMC9841923
- DOI: 10.1016/j.freeradbiomed.2022.09.015
α-Syn overexpression, NRF2 suppression, and enhanced ferroptosis create a vicious cycle of neuronal loss in Parkinson's disease
Abstract
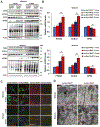
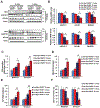
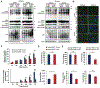
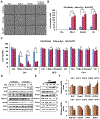
Parkinson's disease (PD) is the second most common neurodegenerative disorder, affecting millions each year. Most PD cases (∼90%) are sporadic, resulting from the age-dependent accumulation of pathogenic effects. One key pathological hallmark of PD progression is the accumulation of alpha-synuclein (α-syn), which has been shown to negatively affect neuronal function and viability. Here, using 3- and 6-month-old Nrf2+/+ and Nrf2-/- mice overexpressing human α-syn (PD model), we show that loss of NRF2 increases markers of ferroptosis across PD-relevant brain regions. Increased ferroptosis was associated with an age- and genotype-dependent increase in α-syn pathology and behavioral deficits. Finally, we demonstrate that α-syn overexpression sensitizes neuronal cells and ex vivo brain slices to ferroptosis induction, which may be due to α-syn decreasing NRF2 protein levels. Altogether, these results indicate that NRF2 is a critical anti-ferroptotic mediator of neuronal survival, and that the vicious cycle of α-syn overexpression and NRF2 suppression, leading to enhanced neuronal ferroptotic cell death, could represent a targetable and currently untapped means of preventing PD onset and progression.
Keywords: Nrf2; Parkinson's disease; aging; alpha-synuclein; ferroptosis.
Copyright © 2022 Elsevier Inc. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

