Development and Validation of an Efficient and Highly Sensitive Enzyme-Linked Immunosorbent Assay for Alemtuzumab Quantification in Human Serum and Plasma
- PMID: 36150715
- PMCID: PMC9819214
- DOI: 10.1097/FTD.0000000000001037
Development and Validation of an Efficient and Highly Sensitive Enzyme-Linked Immunosorbent Assay for Alemtuzumab Quantification in Human Serum and Plasma
Abstract
Background: Alemtuzumab is a humanized monoclonal antibody that targets the CD52 glycoprotein expressed on most lymphocytes, subsequently inducing complement-mediated and antibody-mediated cytotoxicity. Owing to its ability to induce profound immune depletion, alemtuzumab is frequently used in patients before allogeneic hematopoietic stem cell transplantation to prevent graft rejection and acute graft-versus-host disease. In this clinical context, a stable immunoassay with high sensitivity and specificity to determine alemtuzumab levels is essential for performing pharmacokinetic and pharmacodynamic analyses; however, the available methods have several limitations. Here, we report the successful development and validation of an efficient and highly sensitive enzyme-linked immunosorbent assay technique based on commercially available reagents to quantify alemtuzumab in human serum or plasma.
Methods: This enzyme-linked immunosorbent assay technique was developed and validated in accordance with the European Medicines Agency guidelines on bioanalytical method validation.
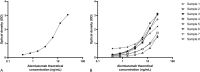
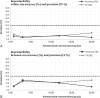
Results: The assay sensitivity (lower limit of quantification) is 0.5 ng·mL -1 , and the dynamic range is 0.78-25 ng·mL -1 . To accommodate quantification of peak concentration and concentrations below the lympholytic level (<0.1 mcg·mL -1 ), patients' serum samples were prediluted 20-400 times according to the expected alemtuzumab concentration. The overall within-run accuracy was between 96% and 105%, whereas overall within-run precision (coefficient of variation) was between 3% and 9%. The between-run assessment provided an overall accuracy between 86% and 95% and an overall coefficient of variation between 5% and 14%.
Conclusions: The developed assay provides accurate insight into alemtuzumab exposure and its effects on the clinical response to treatment, which is key to optimizing treatment strategies.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Association of Therapeutic Drug Monitoring and Clinical Toxicology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Riechmann L, Clark M, Waldmann H, Winter G. Reshaping human antibodies for therapy. Nature. 1988;332:323–327. - PubMed
-
- Buggins AG, Mufti GJ, Salisbury J, et al. . Peripheral blood but not tissue dendritic cells express CD52 and are depleted by treatment with alemtuzumab. Blood. 2002;100:1715–1720. - PubMed
-
- Hale G. The CD52 antigen and development of the CAMPATH antibodies. Cytotherapy. 2001;3:137–143. - PubMed
-
- Wiendl H, Kieseier B. Multiple sclerosis: reprogramming the immune repertoire with alemtuzumab in MS. Nat Rev Neurol. 2013;9:125–126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

