Venous thromboembolism with JAK inhibitors and other immune-modulatory drugs: a Swedish comparative safety study among patients with rheumatoid arthritis
- PMID: 36150749
- PMCID: PMC9887398
- DOI: 10.1136/ard-2022-223050
Venous thromboembolism with JAK inhibitors and other immune-modulatory drugs: a Swedish comparative safety study among patients with rheumatoid arthritis
Abstract
Objective: To assess and compare the incidence of venous thromboembolism (VTE) in patients with rheumatoid arthritis (RA) treated with Janus kinase inhibitors (JAKi), tumour necrosis factor inhibitors (TNFi) or other biological disease modifying antirheumatic drugs (bDMARDs). For contextualisation, to assess VTE incidences in the Swedish general population and in the RA source population.
Methods: We performed a nationwide register-based, active comparator, new user design cohort study in Sweden from 2010 to 2021. The Swedish Rheumatology Quality Register was linked to national health registers to identify treatment cohorts (exposure) of initiators of a JAKi, a TNFi, or a non-TNFi bDMARD (n=32 737 treatment initiations). We also identified a general population cohort (matched 1:5, n=92 108), and an 'overall RA' comparator cohort (n=85 722). Outcome was time to first VTE during the follow-up, overall and by deep vein thrombosis (DVT) and pulmonary embolism (PE). We calculated incidence rates (IR) and multivariable-adjusted HRs using Cox regression.
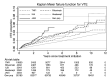
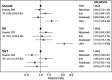
Results: Based on 559 incident VTE events, the age- and sex-standardised (to TNFi) IR (95% CI) for VTE was 5.15 per 1000 person-years (4.58 to 5.78) for patients treated with TNFi, 11.33 (8.54 to 15.04) for patients treated with JAKi, 5.86 (5.69 to 6.04) in the overall RA cohort and 3.28 (3.14 to 3.43) in the general population. The fully adjusted HR (95% CI) for VTE with JAKi versus TNFi was 1.73 (1.24 to 2.42), the corresponding HR for PE was 3.21 (2.11 to 4.88) and 0.83 (0.47 to 1.45) for DVT.
Conclusions: Patients with RA treated with JAKi in clinical practice are at increased risk of VTE compared with those treated with bDMARDs, an increase numerically confined to PE.
Keywords: Antirheumatic Agents; Arthritis, Rheumatoid; Cardiovascular Diseases; Epidemiology.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Karolinska Institutet, with JA as principal investigator, has or has had research agreements with Abbvie, Astra-Zeneca, BMS, Eli Lilly, Galapagos, MSD, Pfizer, Roche, Samsung Bioepis, Sanofi, and UCB, mainly in the context of safety monitoring of biologics via ARTIS/Swedish Biologics Register.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

