Surround Inhibition Mediates Pain Relief by Low Amplitude Spinal Cord Stimulation: Modeling and Measurement
- PMID: 36150892
- PMCID: PMC9536854
- DOI: 10.1523/ENEURO.0058-22.2022
Surround Inhibition Mediates Pain Relief by Low Amplitude Spinal Cord Stimulation: Modeling and Measurement
Abstract
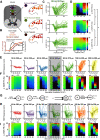
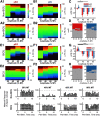
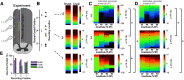
Low-frequency (<200 Hz), subperception spinal cord stimulation (SCS) is a novel modality demonstrating therapeutic efficacy for treating chronic neuropathic pain. When stimulation parameters were carefully titrated, patients experienced rapid onset (seconds-minutes) pain relief without paresthesia, but the mechanisms of action are unknown. Using an integrated computational model and in vivo measurements in urethane-anesthetized rats, we quantified how stimulation parameters (placement, pulse width, frequency, and amplitude) influenced dorsal column (DC) axon activation and neural responses in the dorsal horn (DH). Both modeled and recorded DC axons responded with irregular spiking patterns in response to low-amplitude SCS. Maximum inhibition of DH neurons occurred at ∼80% of the predicted sensory threshold in both modeled and recorded neurons, and responses were strongly dependent on spatially targeting of stimulation, i.e., the complement of DC axons activated, and on stimulation parameters. Intrathecal administration of bicuculline shifted neural responses to low-amplitude stimulation in both the model and experiment, suggesting that analgesia is dependent on segmental GABAergic mechanisms. Our results support the hypothesis that low-frequency subperception SCS generates rapid analgesia by activating a small number of DC axons which inhibit DH neuron activity via surround inhibition.
Keywords: computational modeling; in vivo recording; neuropathic pain; spinal cord stimulation.
Copyright © 2022 Gilbert et al.
Figures





Similar articles
-
Modulation of activity and conduction in single dorsal column axons by kilohertz-frequency spinal cord stimulation.J Neurophysiol. 2017 Jan 1;117(1):136-147. doi: 10.1152/jn.00701.2016. Epub 2016 Oct 19. J Neurophysiol. 2017. PMID: 27760823 Free PMC article.
-
Low-intensity, Kilohertz Frequency Spinal Cord Stimulation Differently Affects Excitatory and Inhibitory Neurons in the Rodent Superficial Dorsal Horn.Neuroscience. 2020 Jan 21;428:132-139. doi: 10.1016/j.neuroscience.2019.12.031. Epub 2020 Jan 7. Neuroscience. 2020. PMID: 31917342
-
Network model of nociceptive processing in the superficial spinal dorsal horn reveals mechanisms of hyperalgesia, allodynia, and spinal cord stimulation.J Neurophysiol. 2023 Nov 1;130(5):1103-1117. doi: 10.1152/jn.00186.2023. Epub 2023 Sep 20. J Neurophysiol. 2023. PMID: 37727912
-
Mechanisms of spinal cord stimulation for the treatment of pain: Still in the dark after 50 years.Eur J Pain. 2019 Apr;23(4):652-659. doi: 10.1002/ejp.1336. Epub 2018 Dec 3. Eur J Pain. 2019. PMID: 30407696 Free PMC article. Review.
-
Spinal Cord Stimulation: Clinical Efficacy and Potential Mechanisms.Pain Pract. 2018 Nov;18(8):1048-1067. doi: 10.1111/papr.12692. Epub 2018 Apr 23. Pain Pract. 2018. PMID: 29526043 Free PMC article. Review.
Cited by
-
Real-world outcomes of single-stage spinal cord stimulation in chronic pain patients: A multicentre, European case series.Interv Pain Med. 2023 Jun 24;2(3):100263. doi: 10.1016/j.inpm.2023.100263. eCollection 2023 Sep. Interv Pain Med. 2023. PMID: 39238903 Free PMC article.
-
Clinical utilization of fast-acting sub-perception therapy (FAST) in SCS-implanted patients for treatment of mixed pain.Interv Pain Med. 2022 Dec 1;1(4):100165. doi: 10.1016/j.inpm.2022.100165. eCollection 2022 Dec. Interv Pain Med. 2022. PMID: 39238875 Free PMC article.
-
Improved Outcomes and Therapy Longevity after Salvage Using a Novel Spinal Cord Stimulation System for Chronic Pain: Multicenter, Observational, European Case Series.J Clin Med. 2024 Feb 14;13(4):1079. doi: 10.3390/jcm13041079. J Clin Med. 2024. PMID: 38398392 Free PMC article.
-
Model-based analysis of subthreshold mechanisms of spinal cord stimulation for pain.J Neural Eng. 2023 Nov 9;20(6):066003. doi: 10.1088/1741-2552/ad0858. J Neural Eng. 2023. PMID: 37906966 Free PMC article.
-
Closed-Loop Spinal Cord Stimulation in Chronic Pain Management: Mechanisms, Clinical Evidence, and Emerging Perspectives.Biomedicines. 2025 Apr 30;13(5):1091. doi: 10.3390/biomedicines13051091. Biomedicines. 2025. PMID: 40426918 Free PMC article. Review.
References
-
- Barchini J, Tchachaghian S, Shamaa F, Jabbur SJ, Meyerson BA, Song Z, Linderoth B, Saadé NE (2012) Spinal segmental and supraspinal mechanisms underlying the pain-relieving effects of spinal cord stimulation: an experimental study in a rat model of neuropathy. Neuroscience 215:196–208. 10.1016/j.neuroscience.2012.04.057 - DOI - PubMed
-
- Blakemore C, Carpenter RHS, Georgeson MA (1970) Lateral inhibition between orientation detectors in the human visual system. Nature 228:37–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
